Chemical Kinetics: What’s the Difference Between a Catalyst and an Intermediate? Study Guide
INTRODUCTION
The curdling of milk is a natural phenomenon. It is very common in our households when the milk is left out in the open for a long time. Curdled milk is used to make cheese. But the industrial level manufacturing of cheese cannot rely on waiting for the milk to curdle. So, what do you think they do? 🤔They induce curdling in the milk by adding a catalyst or a reagent that enhances the speed of the process. Similar is the case with chemical reactions! When we need to increase the reaction speed, we simply add a catalyst to the reaction.
CATALYSTS
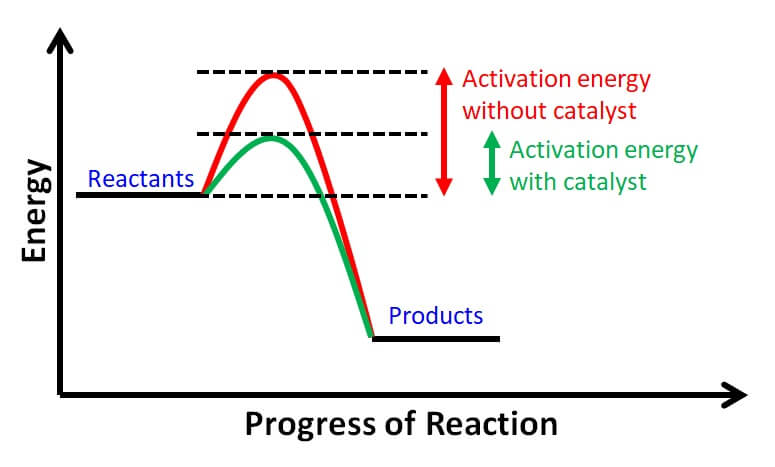
- In chemistry, a catalyst is any material that speeds up a process without being utilized.
- In general, catalytic activity is a chemical interaction between a catalyst and a reactant that produces chemical intermediates that can react more rapidly with each other or with another reactant to produce the desired end product.
- The catalyst is replenished during the reaction between the chemical intermediates and the reactants.
- Several chemical manufacturing processes rely on catalyzed reactions.
INTERMEDIATES
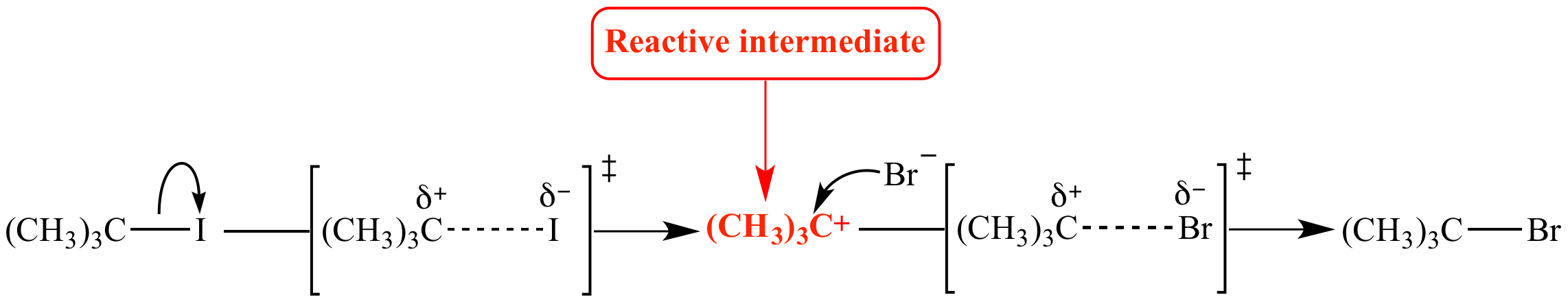
- A reaction intermediate, also known as an intermediate, is a molecular unit generated from reactants that interacts further to produce the immediately seen products of a chemical reaction.
- Most chemical reactions are sequential, meaning they require more than one basic step to finish. Except for the penultimate stage, which produces the final product, each phase produces an intermediate.
- Reactive intermediates are hardly ever segregated and generally have a brief life span. They also do not stay in the product combination due to their limited lifespan.
CONCLUSION
- Catalyst is any material that speeds up a process without being utilized.
- A reaction intermediate is a molecular unit generated from reactants that interacts further to produce the immediately seen products of a chemical reaction.
- The catalyst is replenished during the reaction between the chemical intermediates and the reactants.
FAQs:
1. What is a catalyst in chemistry?
A catalyst is any material that speeds up a process without being depleted.
2. Do catalysts affect intermediates?
Catalysts often react with one or more reactants to produce intermediates, providing the ultimate reaction product renewing the catalyst in the procedure. So yes, catalysts determine which intermediate will be formed in a reaction.
3. What are the differences between an intermediate and a transition state?
The difference between an intermediate and a transition state is that an intermediate has a discontinuous duration (ranging from a few nanoseconds to several days). However, a transition state only lasts for one bond vibration cycle. An intermediate might be a very unstable compound (an unstable intermediate) or a stable compound (a non-reactive intermediate).
4. Is a catalyst the same as an intermediate?
At the start of the process, a catalyst is employed, and it is then regenerated at completion. During the process, an intermediate is formed, but it vanishes at completion. Thus a catalyst is not the same as an intermediate.
We hope you enjoyed studying this lesson and learned something cool about Chemical Kinetics: What’s the Difference Between a Catalyst and an Intermediate! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun, VR classrooms – we promise, it makes studying much more fun! 😎
SOURCES:
- Chemical Kinetics: What’s the Difference Between a Catalyst and an Intermediate?. https://www.ck12.org/c/chemistry/reaction-intermediate/lecture/Chemical-Kinetics:-Whats-the-Difference-Between-a-Catalyst-and-an-Intermediate/. Accessed 7 Feb 2022.
- Catalyst. https://www.britannica.com/science/catalyst. Accessed 7 Feb 2022