Electron Study Guide
INTRODUCTION
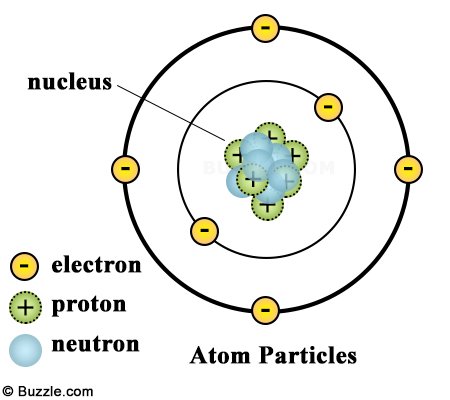
The atom is the smallest unit of matter that occupies space and has mass. Its structure consists of a dense nucleus surrounded by one or more negatively charged particles called electrons. The nucleus, found at the center of the atom, is composed of particles called protons and neutrons. Protons are positively charged and neutrons have no charge, rendering the overall charge of the nucleus positive. Electrons are generally found within a series of shells called orbitals that surround the nucleus. They’re the large determinate Let’s talk about them in more detail.
WHAT ARE ELECTRONS?
An atom is neutrally charged because it contains an equal number of protons and electrons, the opposing charges of which cancel one another out. A unit of electric charge measures up to 1.602176634 × 10⁻¹⁹ coulombs, which is equivalent to the charge of a single electron. When compared to the mass of a proton or neutron, the mass of an electron is practically negligible, weighing in at an infinitesimally small 9.1093837015 × 10⁻³¹ kg. The mass of an electron is so slight, in fact, that it is not considered while calculating the mass of an atom.
WHAT ARE CATIONS AND ANIONS?
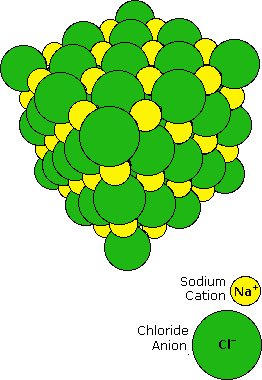
Whenever an atom has an unequal number of protons and electrons, it becomes a positive or negative ion depending on which particle is more abundant. A positively charged Atoms possessing more protons than electrons are called positively charged ions or cations. For example – Al₃ , Ca₂. Whereas negatively charged ions or anions are those atoms with more electrons than protons. For example – Cl, Br.
HOW DO ELECTRONS ASSIST IN THE FORMATION OF BONDS?
Electrons that are situated in concentric shells around the nucleus are closer to the nucleus and are held tightly due to the attractive force of the nucleus, while the ones away from it are quite loosely attached. They are responsible for creating bonds between different atoms. Electrons can assist in the formation of two types of chemical bonds, electrovalent and covalent bonds.
In an electrovalent bond, one atom loses its electrons to become a positively charged ion, and another atom gains the same electrons to become a negatively charged ion. When a number of electrons are shared between two atoms where neither gains nor loses any, the bond formed is known as a covalent bond.
FAQs
1. What is the mass and charge of an electron?
The mass of an electron is very less and measures up to 9.1093837015×10-31 kg, while a unit of electric charge measures up to 1.602176634×10-19 coulomb.
2. How does an atom become charged?
The amount of protons and electrons present in an atom determines the type of its electric charge. If the number of protons present in the nuclei is more than the number of electrons revolving around the nuclei, then it is a positively charged ion or a cation. Again, if the number of electrons surpasses the number of protons, then it becomes a negatively charged ion or an anion.
3. Where is an electron?
An atom can be structurally divided into the nucleus and the shells. The shells are concentric circles around the nucleus and are home to the electrons.
4. Why do electrons repel each other?
Since like charge, repels, and all electrons are negatively charged, they are constantly repulsed from each other. But, the attractive force of the nucleus holds them together in an atom.
We hope you enjoyed studying this lesson and learned something cool about Electrons! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES
- The Electron- Crash Course: https://www.ck12.org/c/chemistry/electron/lecture/The-Electron:-Crash-Course/?referrer=concept_details. Accessed 7th March 2022.
- Electrons: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/04%3A_Atomic_Structure/4.08%3A_Electrons. Accessed 7th March 2022.
- Electron Definition: Chemistry Glossary: https://www.thoughtco.com/definition-of-electron-chemistry-604447. Accessed 7th March 2022.