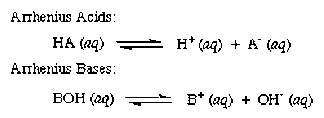Arrhenius Acid Study Guide
Acids and bases can be found in anything from the vinegar in your kitchen cabinet to your bathing soap. What does that really mean to say something is acidic or basic, though? To address this topic, we must first look at some of the ideas that describe acids and bases. The Arrhenius theory will be the topic of this article.
ARRHENIUS ACID
- Svante Arrhenius, a Swedish chemist, first presented the Arrhenius hypothesis of acids and bases in 1884. He proposed categorizing certain substances as acids or bases depending on the type of ions generated when they were combined with water.
-
Any species that raises the concentration of Hydrogen ions or protons in an aqueous solution is known as an Arrhenius acid. Understand the dissociation reaction of hydrochloric acid in the water, for instance:
HCl(aq) → H+(aq) Cl-(aq) -
HCl disintegrates into H ions and Cl- ions in an aqueous solution of HCl. Hydrochloric acid is an Arrhenius acid because it causes a rise in the concentration of H ions in the solution.
- Ionizability does not apply to all hydrogen atoms in molecular compounds.
- The hydrogen atoms in methane (CH₄) are covalently bound to carbon in just slightly polar bonds.
- Methane has no acidic characteristics since the atoms are incapable of ionization.
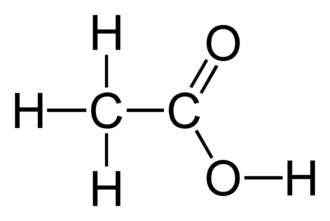
- Organic acids, such as acetic acid (CH₃COOH), are a type of acid. The molecule contains four hydrogen atoms; however, only the one hydrogen atom linked to an oxygen atom is ionizable.
Hydrogen or Hydronium Ions
• The symbols H+ and H3O+ are used to represent the hydrogen and hydronium ions, respectively.
• The hydrogen atom’s electron is taken out to create a hydrogen ion. Due to its high reactivity, this reacts with water in an aqueous environment to generate a hydronium ion.
• The protonation of water also produces hydrogen ions.
• Ions of hydrogen are less stable than ions of hydrogen.
What is an Acid according to Arrhenius
The term “Arrhenius acid” refers to a material that contains a hydrogen atom and readily releases a hydrogen ion or proton when it is in contact with water. For instance, chloride and hydronium ions are created when hydrochloric acid is dissolved in water. In an aqueous solution, acetic acid also functions as an Arrhenius acid by forming the acetate ion and the hydronium ion.
Corrosive toxins typically affect the eyes and lungs. Burns caused by acids or bases may potentially harm the cornea. The respiratory system is corroded by air contaminants such as sulphur oxides, nitrogen oxides, chlorine, and ammonia. The lining of the nose, sinuses, and lungs are primarily affected.
Basicity of Arrhenius acids
The quantity of replaceable hydrogen ions in an acid is referred to as the basicity of an Arrhenius acid. For instance, the basicity of phosphoric acid is three because it ionizes in its aqueous solution to produce three hydrogen ions.
Example: H3PO4 (Tribasic acid (Basicity = 3)
Arrhenius Acid as Strong Electrolytes
When Arrhenius sought to understand why some solutions could carry an electric current, he discovered that conductivity resulted from the presence of ions. He noticed that the chemicals HCl, HNO3, and H2SO4 act as potent electrolytes when dissolved in water. Ionization processes in water produced this.
These compounds are known as strong acids because they are powerful electrolytes that generate H+ ions. Ionization processes in water produced this.
List of Arrhenius Acids
The most stringent hypothesis is the Arrhenius acid-base theory, which demands that the chemical contain an H+ or OH- and be soluble in water.
The list of Arrhenius acids is given below:
| Acid | Formula |
|---|---|
| Perchloric | HClO |
| Hydroiodic | HI |
| Hydrochloric | HCl |
| Nitric | HNO3 |
| Sulfuric | H2SO4 |
| Iodic | HIO3 |
| Oxalic | H2C2O4 |
| Hydrobromi | HBr |
TYPES OF ACID ON THE BASIS OF THE NUMBER OF HYDROGEN IONS RELEASED
- On the basis of the number of hydrogen ions released in the aqueous solution, acids can be classified as monoprotic or polyprotic.
- A monoprotic acid is the one that has one ionizable hydrogen in it. Monoprotic acids include hydrochloric acid and acetic acid. An acid with numerous ionizable hydrogens is known as a polyprotic acid.
Limitations of the Arrhenius Definition
The Arrhenius hypothesis only applies to substances that are dissolved in water. It only counts as bases those compounds that release hydroxide ions in aqueous solutions and only count as acids those substances that release hydrogen ions in aqueous solutions. The theory is unable to account for the neutralization exception. Since the Arrhenius concept was the first inference made, it had a number of problems because it was developed so long ago, before additional ideas about chemistry had been put into consideration.
CONCLUSION
- In the aqueous phase, an Arrhenius acid is a chemical that ionizes to produce hydrogen ions (H).
- On the basis of the number of hydrogen ions released in the aqueous solution, acids can be classified as monoprotic (one hydrogen ion) or polyprotic (more than one hydrogen ion).
FAQs:
1. What is an Arrhenius acid?
According to Arrhenius, acids are hydrogen-containing substances that dissociate in water to give H ions or protons, while bases are hydroxide compounds that dissociate to give OH ions.
2. What is the definition of an Arrhenius acid? Give an example.
According to the Arrhenius hypothesis, an Arrhenius acid is a chemical that contains hydrogen atoms and may easily produce hydrogen ions or protons in its aqueous solution. When hydrochloric acid is dissolved in water, it produces the ions chloride (Cl-) and hydronium (H₃O).
We hope you enjoyed studying this lesson and learned something cool about Arrhenius Acid! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
]]>