Neutralization Reaction Study Guide
INTRODUCTION
What is one of a referee’s most significant characteristics? An impartial referee (neutral) is essential. They can’t provide one team an advantage over the other. Being neutral in chemistry implies not being an acid or a basic. A neutral material is, for instance, pure water. An acid and a base interact to generate neutral products, such as water, in several chemical processes. As you read this article, you’ll see how it all transpires.
NEUTRALIZATION REACTION
A neutralization reaction is a chemical reaction that occurs when an acid and a base react quantitatively to produce water and salt as products.
Before we go further in this topic, let’s understand what acid and base are.
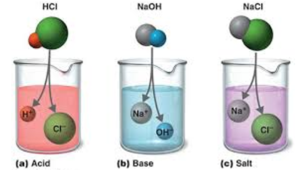
Acid: When an acid dissolves in water, it creates positive hydrogen ions (H ) and negative non-metallic ions. (Ions are atoms that have gained or lost electrons and have become charged.) A good example of an acid is hydrochloric acid (HCl).
Base: A base is a compound that yields negative hydroxide ions (OH-) and positive metal ions when dissolved in water.
Relation Between the Strength of Reactants and Resultant pH
The ionization and pH drop of an acid determine its strength.The acidity increases as the pH decreases. The acidity decreases as the pH rises. The basicity rises as a result. With increasing acid content, an acidic solution’s pH changes. The amount of hydrogen ions created depends on the acid content. As an illustration, pH readings may be used to compare the acidity of various acids. The concentration of each acid that will be compared must be the same.
The pH scale has a range of 0 to 14, with 7 representing neutrality, everything below 7 being acidic, and anything over 7 being basic. The pH scale classifies a substance as acidic if it has a value lower than seven. This is connected to the pH’s level of acidity (strong or mild acidity). A scale called pH may be used to determine how acidic or basic a solution is. Numbers on the scale range from 1 to 14. The pH scale considers 7 to be neutral. The pH of pure water is supposedly 7. Acids are represented on the pH scale in groups of 1-6.
ACID-BASE INTERACTION
Acid + base(alkali) → salt + water
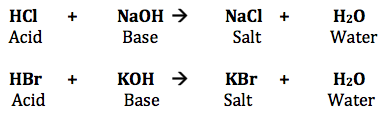
A neutral solution is formed when equal quantities of a strong acid, such as hydrochloric acid, are combined with a strong base, such as sodium hydroxide. The reaction’s products don’t have the properties of an acid or a base. The balanced chemical equation is shown below.
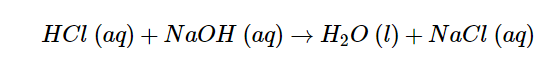
A net ionic equation is a more precise representation of chemical processes that occur in an aqueous solution. The following is the complete ionic equation for the neutralization of hydrochloric acid by sodium hydroxide:
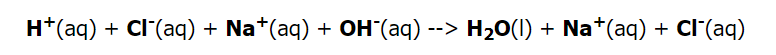
Because the acid and base both are strong, they are totally ionized and, like the NaCl generated, as a result, are recorded as ions. The sodium and chloride ions are spectator ions in the process; hence the net ionic reaction is as follows.
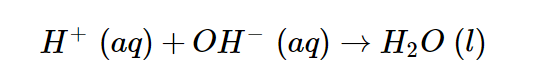
The net ionic reaction of hydrogen ions interacting with the hydroxide ion to form water simplifies all neutralization reactions of a strong acid with a strong base.
Application of Neutralization Reaction
A neutralizing reaction has the following applications:
• Because the crop cannot flourish in acidic soil, it is used as fertilizer.
• The effects of the formic acid that a bee sting has generated in our systems can be mitigated by baking soda.
• To combat the effects of the HCl generated in our stomachs, we utilize antacid pills that contain magnesium oxide when we have acid reflux illness.
Titration Methods
Chemical titration is a technique used to determine unknown acid or base concentrations by determining the neutralization point. Utilizing a pH indicator or pH meter, we can locate the location where neutralization takes place. The molarity of the unknown particle may be determined using straightforward stoichiometric calculations and knowledge of the volume and molarity of the known substance.
Wastewater Treatment
The majority of the trash that is produced as industrial effluents has a considerable amount of toxicity that is bad for the environment. Therefore, before disposing of them, we must reduce their toxicity. A variety of compounds are utilized, depending on the application. Sodium bicarbonate, magnesium oxide, calcium oxide, and calcium carbonate are a few typical examples.
Nanomaterial Synthesis
The heat produced by a neutralization reaction is employed to aid in the chemical reduction of metal precursors.
In our Digestive Systems
Antacids neutralize the stomach acid that is produced. They are frequently used in cases where neutralizing stomach acid is beneficial. Taking heartburn-causing acid reflux as an illustration. Antacid users often don’t experience any negative side effects.
Controlling Soil pH
In soil that has a specific pH level, plants can flourish. Acidic soil cannot support the growth of plants. Therefore, bases are added to the soil to mitigate its effects. The soil is treated with substances to lower its pH, including burnt wood ashes, limestone, and powdered lime. By reducing the impact of soil acids, this method aids in regulating the pH of the soil.
Types of Neutralization Reactions
-
Strong acid and strong base: In a strong acid-strong base titration, the acid and base will combine to form a neutral solution. The reaction’s equivalence point, which results in a pH of 7, will see the combination of hydronium and hydroxide ions to create water. This is valid for each and every titration involving powerful acids and powerful bases.
-
Strong acid and weak base: Strong acids completely split into their ions in water, whereas weak acids only partially do so. Since there are only seven strong acids, memorizing them is a popular choice. All further acids are weak. Perchloric acid, chloric acid, nitric acid, sulfuric acid, hydrobromic acid, and hydroiodic acid are some of the potent acids.
-
Weak acid and strong base: In a process known as weak acid-strong base neutralization, a weak acid reacts with a strong base to create a basic salt and water. This type of reaction, which takes place when acetic acid and NaOH mix to form sodium acetate, is an illustration of water. Due to the produced acetate ion’s propensity to pull protons from the water, the hydroxyl ions are released.
-
Weak acid and weak base: In the weak acid-weak base neutralization process, a weak acid reacts with a weak base to generate a neutral salt and water. The interaction between water and acetic acid, which results in the creation of ammonium acetate, is an example of this type of reaction. Weak bases and acids do not completely dissociate, hence the equilibrium is to the left in this situation. A weak acid is one that partially separates into its ions in water or an aqueous solution. A strong acid, on the other hand, totally dissociates into its ions in water.
CONCLUSION
- A neutralization reaction is a chemical reaction that occurs when an acid and a base react quantitatively to produce water and salt as products.
- A net ionic equation is a more precise representation of chemical processes that occur in an aqueous solution.
FAQs
1. What is the net ionic reaction for the neutralization reaction between an acid and a base?
When a strong acid, as well as a strong base, are combined, they react using the net-ionic equation below:
H₃O⁺(aq) + OH⁻(aq) → 2H₂O(l)2. Which is the net ionic equation for the neutralization reaction of a strong acid with a weak base?
When a weak base and a strong acid are combined, they react as follows:
B(aq.) H₃O (aq.) → HB (aq.) H₂O (l)The pH of the resultant solution may be estimated by studying the equilibrium interaction of HB with water if the acid and base are equimolar.
We hope you enjoyed studying this lesson and learned something cool about Neutralization Reaction! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise, it makes studying much more fun! 😎
]]>