Discovery Of Electrons Study Guide
Introduction
Dalton’s atomic theory was crucial in the development of contemporary atomic theory. However, one of his fundamental assumptions was eventually proven to be false.
Atoms, according to Dalton, are the tiniest units of matter, consisting of tiny, hard spheres that cannot be subdivided anymore. This belief remained until physics investigations revealed that the atom was made of much smaller particles.
The first basic particle found was the electron. We’ll go over the significant experiment that led to the discovery of the electron in this article.
THE DISCOVERY OF ELECTRON
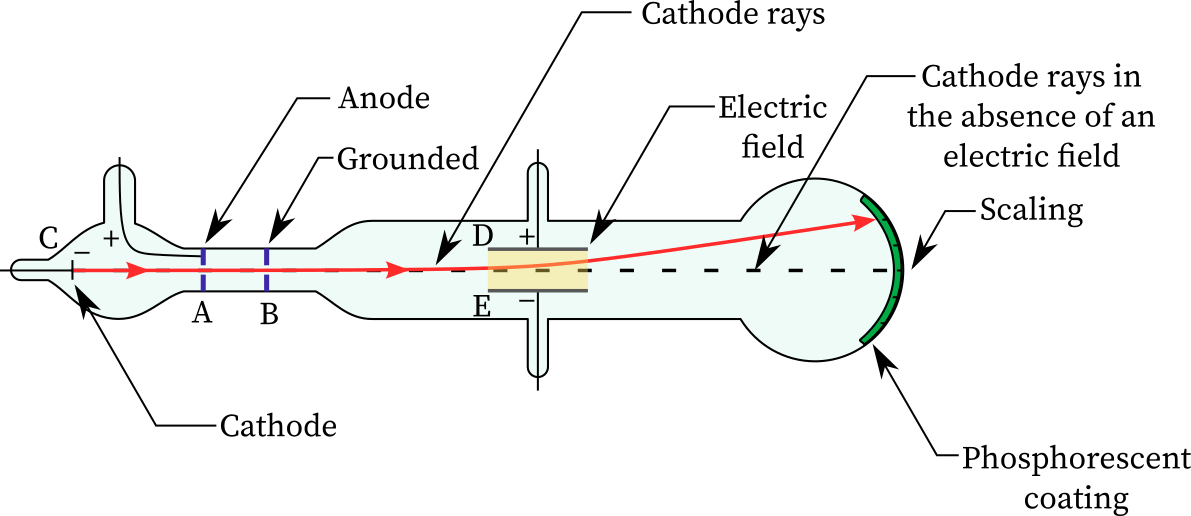
- In the early 1900s, physicist J.J. Thomson began research on cathode ray tubes.
- Cathode ray tubes are glass cylinders that have been vacuum-sealed and have had the majority of the air evacuated.
- A high voltage is applied to two electrodes at one end of the tube, forcing particles to flow from the cathode (negatively charged electrode) towards the anode (positively charged electrode).
- The tubes are called cathode-ray tubes because the particle beam, or cathode ray, begins at the cathode.
- Coating phosphors on the tube’s extremity, beyond the anode, can help identify the beam. The phosphors flash or generate light when the cathode ray touches them.
- To explore the particles’ properties, Thomson enclosed the cathode ray with oppositely charged electrical panels.
- From the negatively charged electric panel to the positively charged panel, the cathode ray was diverted.
- According to this, the cathode ray was composed of negatively charged particles.
- Thomson also placed two magnets along either side of the cylinder, observing that the cathode ray was redirected by the magnetic field.
- Thomson utilized the results of these experiments to compute the mass-to-charge proportion of cathode ray particles, which revealed an unexpected result: each particle’s mass was far, far lower than any existing atom.
- Thomson tested a variety of metals as electrode materials and observed that the cathode ray’s properties remained similar independent of the cathode material.
Based on the facts, Thomson came to the following conclusions:
- The cathode ray is made up of negatively charged particles.
- Each particle must be a member of the atom since its mass is 1/2000’th that of a hydrogen atom.
- These subatomic particles could be observed in the atoms of all elements.
Thomson’s findings were initially controversial, but experts gradually accepted them. Electrons became the more frequent moniker for his cathode ray particles. The electron’s discovery refuted Dalton’s atomic theory’s notion that atoms were indivisible.
SUMMARY
- The very first fundamental particle found was the electron.
- Thomson tested a variety of metals as electrode materials and observed that the cathode ray’s properties remained similar independent of the cathode material.
FAQS:
1. What did the discovery of the electron disprove?
The discovery of the electron undermined Dalton’s atomic theory’s assumption that atoms were indivisible. To compensate for the existence of electrons, a whole new atomic model was necessary.
2. What was used to discover an electron?
J. J. Thomson discovered the presence of electrons during the course of three experiments. He achieved this by utilizing a cathode ray tube, which is a vacuum-sealed tubular structure with a cathode and anode along one end that produces an electron beam that travels to the other end.
3. Which gas is used in the discovery of electrons?
An evacuated container is filled with hydrogen gas for better outcomes in a cathode tube experimentation.
We hope you enjoyed studying this lesson and learned something cool about the Discovery Of The Electron! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise, it makes studying much more fun!😎
SOURCES:
- Discovery of the Parts of The Atom:https://courses.lumenlearning.com/physics/chapter/30-2-discovery-of-the-parts-of-the-atom-electrons-and-nuclei/ accessed 14 march 2022
- Discovery of Electron: https://byjus.com/chemistry/discovery-of-electron/ accessed 14 march 2022
- Electronic Structure of Atoms: https://www.khanacademy.org/science/chemistry/electronic-structure-of-atoms/history-of-atomic-structure accessed 14 march 2022