Octet Rule Study Guide
INTRODUCTION
Have you ever wondered how chemical reactions occur? How does the bonding between two atoms become strong? Or what is the mechanism behind covalent bonding? All the question has one answer, the atoms want to stabilize their structure; hence, they follow the octet rule. Now, what is the octet rule in chemistry? Let’s find out the meaning of the octet rule together!
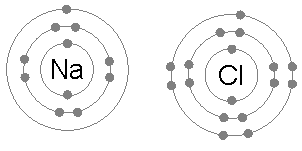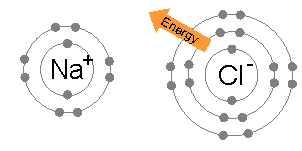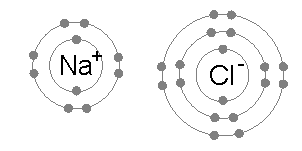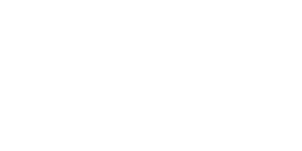
OCTET RULE CHEMISTRY DEFINITION
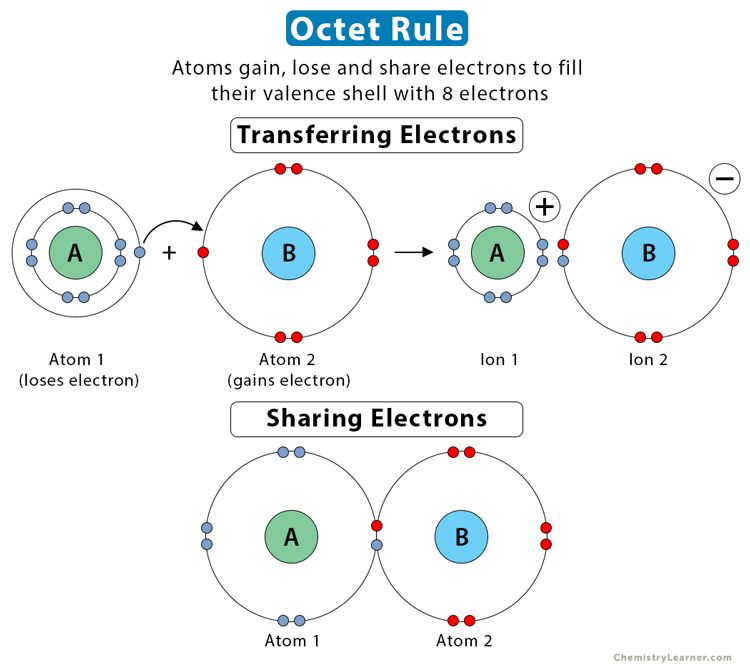
Every atom wants to attain stability by either losing its lone electron or gaining a new electron to attain the configuration of the nearest noble gas. This phenomenon is known as the octet rule.
Noble gases do not like to share their electrons as they are chemically inert. They have attained the octet rule and are hence stable. The molecules like halogens, oxygen, etc., attain the octet rule. Besides, the s-block elements, as well as p-block elements that follow the octet rule, will obey to reach the stability of their atoms.
EXCEPTION OF THE OCTET RULE
Not every element follows the octet rule; there are some exceptions to this rule. They are as follows:
- An atom, ion, or molecule with an unpaired valence electron known as a free radical does not follow this rule. Although, these elements or atoms or ions are very unstable and break down easily.
- Some elements like helium, lithium, and hydrogen obey the duet rule as the first shells only contain 2 atoms. Hence, if they achieve 2 atoms in their valence shell, they are stable.
- The transition elements do not obey the octet rule as they have a d-orbital present. The valence shells can hold 18 electrons.
- The aromatic compounds follow Heckle’s Law due to their delocalization of pi-electrons. They do not follow the octet rule.
Limitations of Octet Rule
The octet rule is generally obeyed by the elements of second and third periods with the following exceptions.
- The Incomplete Octet – Species in which an atom does not achieve an octet of electrons are less common than hypervalent compounds, but by no means uncommon. These are known as incomplete-octet compounds.
__Example – BeCl2 __
The requirement of electrons is 8 to follow the octet rule. In this case the beryllium has only 4 electrons and the shell is incomplete which eventually means that beryllium does not follow the octet rule.
-
Odd Electron Molecules – All atoms of a compound containing odd number of electrons will not satisfy octet rule as even number of electrons are required for pairing of electrons.
__Example – NO __
In the case of Nitric Acid, nitrogen has only three electrons, which is an odd number, so it has paired with oxygen, which has six electrons, which is an even number. Even after pairing, the total number of electrons in nitrogen is 7, which is less than the number of electrons required to follow octet rule. This demonstrates that nitrogen does not follow the octet rule.
- The Expanded Octet – Elements of third period and beyond can accommodate more than 8 electrons due to the availability of vacant d orbitals.
Example – PCl5
In the case of phosphorous pentachloride, the phosphorous has 10 electrons, whereas the octet rule requires only 8 electrons. This demonstrates that phosphorous does not obey the octet rule.
FAQs
1. What is the simple definition of the octet rule?
The octet rule states that atoms gain or lose electrons to achieve an outer shell electron structure nearest that of a noble gas. The attractive force between atoms is informally calculated with this rule.
2. What is the octet rule, and what is its importance?
The octet rule is a rule which states the behavior of an atom to attain stability. The atoms try to gain or lose electrons to attain an outer shell electron configuration nearest that of a noble gas.
The octet rule is crucial in covalent bonding simply because both atoms get a full valence shell due to the sharing electrons. All atoms seek to reach a full valence shell, like those present in noble gases. Wondering why? This is considered the most stable electron structure.
3. What is the octet rule?
The octet rule states that atoms are most stable when their valence shells are loaded with eight electrons. The atoms want to have an outer shell configuration of noble gas so that they can attain stability.
We hope you enjoyed studying this lesson and learned something cool about the Importance of the Octet Rule! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
]]>