Reactants and Products Study Guide
We are surrounded by chemical reactions and their results at all times. Whether eating food or going out for a jog to maintain your fitness, each of these activities involves some kind of chemical reaction. Food is converted to insoluble solid through chemical reactions whereas sweating is also an exothermic chemical reaction.
These chemical reactions are the computations of different compounds or elements present in the environment. We refer to these elements as reactants and products in a chemical reaction.
REACTANTS
Reactants are chemical elements or compounds that react with or combine with another compound or element within a chemical reaction (the process of transformation of chemical substances). These are usually at the beginning of the arrows in chemical reactions. We will now take an example of a chemical equation (symbolic representation of a chemical reaction) to understand the term more effectively.
Hydrogen + Oxygen → WaterThe above reaction is the chemical composition of water. The arrow in a chemical reaction means the direction in which the reaction occurs. If we use the scientific names of the elements of the equation, it would look something like this.
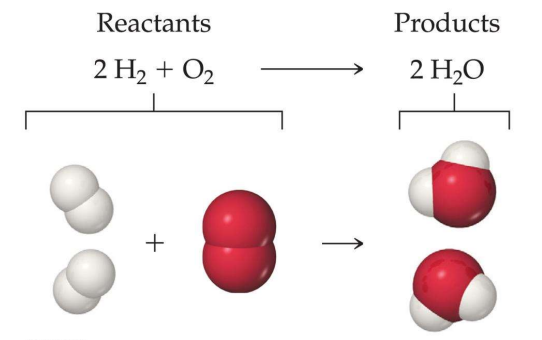
Here we can see that hydrogen and Oxygen are the reactants, and they combine to form a chemical compound H2O, i.e., water.
PRODUCTS
The term product refers to the resultant compounds obtained after the composition or computation of two or more elements. One or more products can be obtained in a single chemical reaction, and products are written at the right-hand side of the arrow in chemical reactions. We can take the same example to understand the concept of a product in a chemical reaction. One important observation is that each reaction needs more than one reactant.
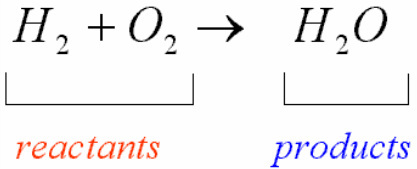
Here, the product we obtain is water. Molecules of both hydrogens and oxygen combine to form a single compound molecule of water. This equation only has one single product as only two chemicals react with each other to form one compound.
CONCLUSION
- Reactants are elements that combine to form simple or complex compounds in a reaction.
- Products are obtained after the reaction between two or more reactants in a reaction.
- Without the involvement of reactants, chemical equations are not possible.
- A single product can be obtained, but there must be two or more reactants for a chemical reaction.
- The arrow in a chemical reaction means the direction in which the reaction occurs.
FAQs:
1. What are reactants and products?
Ans. Reactants are chemical compounds or elements that combine to form products. Products are results obtained after computations of reactants in a chemical equation.
2. What are some characteristics of reactants in a chemical equation?
Ans. Reactants are usually written on the left-hand side, and at least two reactants are necessary for a reaction.
3. What is the least number of products obtained in a chemical equation?
Ans. At least one product is obtained in a chemical equation.
We hope you enjoyed studying this lesson and learned something cool about Reactants and Products! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun, VR classrooms – we promise, it makes studying much more fun! 😎
]]>