Atomic Radius Study Guide
INTRODUCTION
Who doesn’t enjoy strolling down a Walmart aisle, gazing into the cupboards brimming with their favorite snacks? The packs are already placed so that they cover the most surface area possible. The number of cereal boxes that can be placed in a cabinet is far less than the number of potato chips packets stored in the same space, which is even less than the number of chocolate bars stored in the same space.
Knowing the dimensions of the objects we’re interacting with can help us determine how much space is required. Even if the atoms are tiny, their radius can be estimated. Also, when attempting to describe the behavior of atoms or compounds, the size of atoms is critical. The atomic radius is one of the ways we can describe the size of atoms.
What is Atomic Radius?
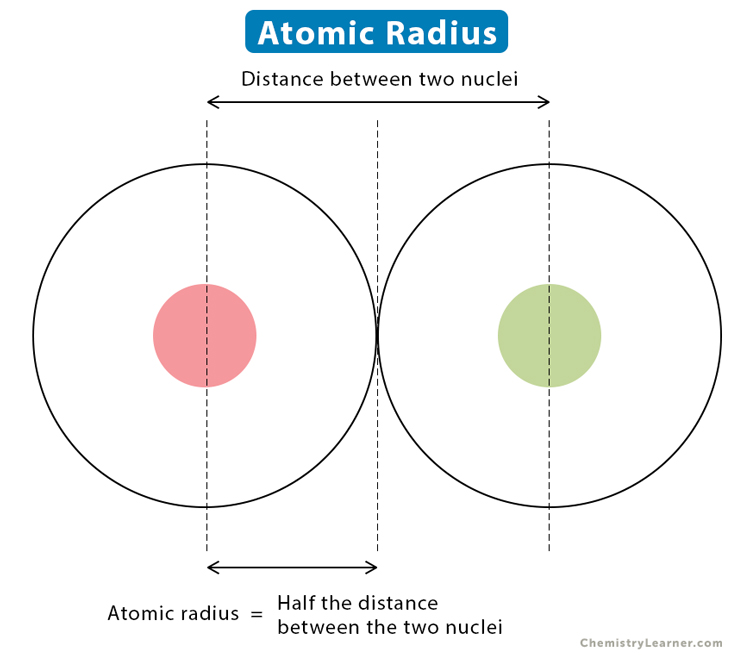
Technically, the atomic radius is the distance between the nucleus’s center and the outermost electron-carrying shell where the electron cloud’s density reaches its greatest. But, orbital limits are hazy and, in reality, vary depending on the circumstances. The distance between the nuclei of two similar atoms linked together is measured to standardize the measurement of atomic radii. Half of the length between the nuclei of the two similar atoms linked together is known as the atomic radius. In the periodic table, this measurement follows a typical pattern.
Ionic Radius
An atom becomes a cation when an electron is lost, and an anion when an electron is gained. The distance between an ion’s nucleus and its outermost shell is referred to as the ionic radius. A cation will have an atomic size that is smaller than its parent atom. The size of an anion is often greater than that of its parent atom. This is because adding electrons to an atom increases the total amount of electrons in the atom, which tends to enhance electron repulsion and mask the net effective nuclear charge.
Lanthanide Contraction
The continuous reduction in the size of the ions and atoms of the rare earth elements from lanthanum (atomic number 57) to lutetium is known as lanthanide contraction (with an atomic number 71). The nuclear charge can become one unit more positive for each subsequent atom, which is accompanied by a commensurate rise in the number of electrons in the 4f orbitals that surround the nucleus.
As the lanthanide elements develop, the 4f electrons poorly shield one another from the rising positive charge of the nucleus, which causes a gradual rise in the effective nuclear charge that attracts every electron and leads to a series of reductions in ionic and atomic radii.
TYPES OF ATOMIC RADIUS
Van der Waals radius, metallic radius, and covalent radius are the three commonly used concepts of atomic radius.
COVALENT RADIUS
The covalent radius, abbreviated as Rcov, is a measurement of the radius of an atom in a covalent bond. Picometres or angstroms are the most common units of measurement.
VAN DER WAALS RADIUS
The radius of an imagined hard-sphere indicating the radius of closest distance for another atom is the Van der Waals radius of an atom.
METALLIC RADIUS
The radius of an atom linked by a metallic connection is known as the metallic radius. The metallic radius is half of the total separation between the nuclei of two adjacent atoms.
ATOMIC RADIUS TREND IN THE PERIODIC TABLE
1. ACROSS A PERIOD
The atomic radius in a periodic table varies as we move across or down the table. A molecule’s atomic radius decreases over time. While the number of electrons keeps increasing, it only adds to the major energy level; hence the electron cloud doesn’t enlarge. However, as time passes, the electrons are closer to the nucleus, which reduces the atomic radius.
Note: The atomic radius rapidly rises as we move from halogens to inert gases.
2. DOWN A PERIOD
Atomic radii increase as you descend a group. This is because electrons in each group inhabit progressively higher energy levels. Atomic radii grow in lockstep with the size of electron clouds.
CONCLUSION
- The space between the nuclei of two similar atoms linked together determines the atomic radius.
- Atoms’ atomic radius shrinks with time as they move from left to right.
- Within a group, the atomic radius of atoms usually increases from top to bottom.
FAQs:
1. Why does the atomic radius decrease across a period?
Effective nuclear charge rises with time as electron shielding stays unchanged. A greater effective nuclear charge attracts electrons more strongly, drawing the electron cloud forward to the nucleus and reducing the atomic radius.
2. What are the two trends in atomic radius?
The two trends in the atomic radius are:
- Atomic radius decreases as we move from left to right.
- The atomic radius increases from top to bottom.
We hope you enjoyed studying this lesson and learned something cool about the Atomic Radius! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
]]>
