Ionic radii Study Guide
INTRODUCTION
Throughout the history of sports, various types of sports balls have been used for various games. Basketballs, tennis balls, handballs, soccer balls, footballs, and a variety of others are among the most well-known. Even though they are balls, they come in a variety of shapes, sizes, and applications. Their size restricts their application to a specific sport. A tennis ball certainly cannot be used to play soccer. Ions and atoms, too, have radii, and many of their characteristics are based on this. In this article, we will discuss more about atomic radii vs ionic radii.
IONIC RADII
Protons and electrons have a high affinity to one another. The size of any molecule is influenced by the strength of the attraction and the relative numbers of the two particles (protons and electrons) in a specific atom or ion.
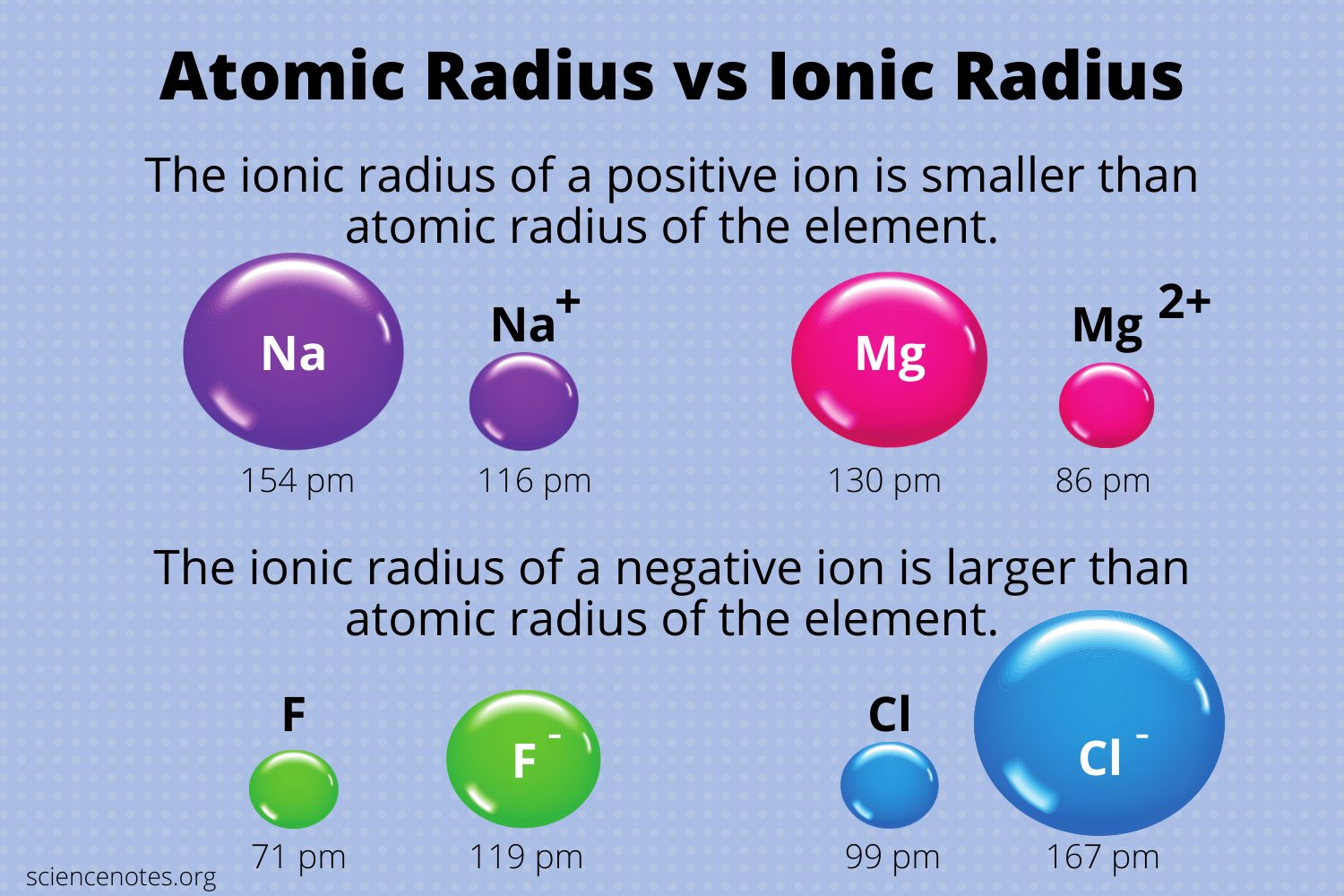
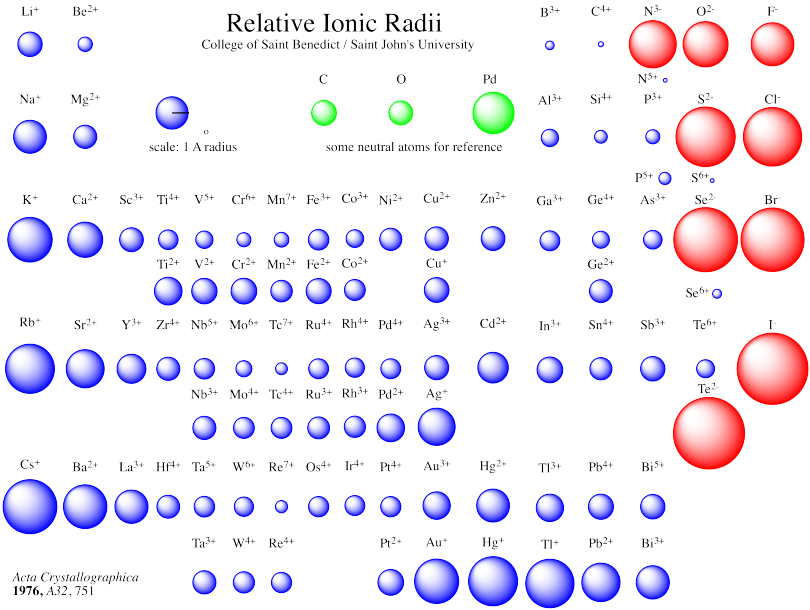
The radius of an ion, whether anion or cation, is called the ionic radius (rion). Although atoms and ions do not have specific borders, it is beneficial to treat them as spherical particles with radii. The spacing between ions in a crystal lattice can be calculated by adding the ionic radii of a cation and an anion. Picometers (pm) or Angstroms (Å) are the most common units to express ionic radii.
REMOVAL OF ELECTRONS
When the valence electron(s) are eliminated, and the resultant ion has one less occupied primary energy level, resulting in a smaller electron cloud. The protons now dominate electrons, and the remaining electrons are drawn closer to the nucleus.
ADDITION OF ELECTRONS
When the electrons exceed the protons, the protons’ total attractive pull for the electrons decreases. Since there are more electrons, there are more electron-electron repulsions, which causes the electron cloud to expand out.
IONIC RADIUS TRENDS
When an atom loses electrons to create a cation, the lost electron no longer shields the other electrons from the nucleus’ charge; as a result, the other electrons are more attracted to the nucleus, and the atom’s radius shrinks. Similarly, when an electron is added to an atom to form an anion, the additional electron resists other electrons, causing the atom to grow in size.
Like other forms of atomic radii, Ionic radii grow as you descend a group and drop as you go across. This only occurs if the elements are of the same ion type, i.e., cations or anions.
CONCLUSION:
- The radius of an atom in a crystal lattice is measured to calculate its ionic radius.
- When electrons are removed, an ion is formed that is smaller than the source element.
- When electrons are added together, they form an ion that is bigger than the source element.
FAQs:
1. Why do ionic radii increase down a group?
The ionic radii increase down a group because there is an extra energy level for electrons as you travel down the group of elements.
2. Why do ionic radii decrease across periods?
The ionic radii decrease across a period because effective nuclear charge rises, consequently decreasing the ionic radii over time.
3. What is ionic radii in chemistry?
The radius of an ion, whether anion or cation, is called the ionic radius.
We hope you enjoyed studying this lesson and learned something cool about the Ionic Radii! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎.
SOURCES:
- Ionic Radius: https://courses.lumenlearning.com/introchem/chapter/ionic-radius/ Accessed 22 Feb 2022
- Periodic Trends: https://www.ck12.org/c/chemistry/periodic-trends%3A-ionic-radii/lesson/Ionic-Radii-CHEM/ Accessed 22 Feb 2022