Importance Of Using An Airbag Study Guide
INTRODUCTION
When cars were first manufactured, there was no way to ensure that the people in them remained protected. Later, seatbelts were attached. But, this was not enough to ensure protection. Thus, airbags were manufactured for additional protection of people riding in vehicles. Let us find out how airbags work.
WHAT ARE AIRBAGS, AND WHY ARE THEY IMPORTANT?
Airbags are inflated bags that serve as a cushion for any part of your body. They are usually found in cars and are attached in a place in front so that they can spring out of their place and cushion you from hard surfaces. They are essential for every vehicle because they play a huge role in protecting you from accidents.
While seatbelts promptly keep you strapped in your place, there is no saying how far your upper body can be injured in the face of an accident. Thus, airbag chemistry proved to be very effective, working alongside seatbelts in the function of protecting the people that travel in vehicles.
HOW IS CHEMISTRY APPLIED IN THE MANUFACTURING OF AIRBAGS?
Airbags contain a compound known as sodium azide, which is quite stable at normal temperature, and no changes occur in the airbag. But, at a higher temperature, this compound breaks down into its subsequent products of sodium and nitrogen. Thus, airbags do not contain air as the name implies but are filled with nitrogen.
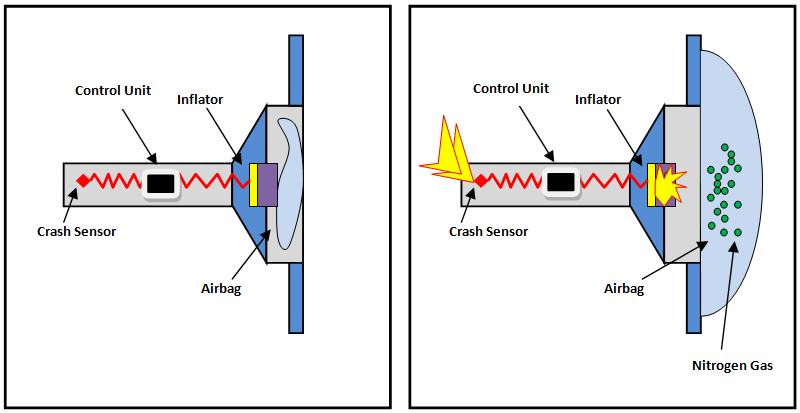
HOW ARE THEY ACTIVATED IN CARS?
Since NaN3 reacts at high temperatures, there are collision sensors attached in front of a car which takes advantage of this property. As soon as a collision is sensed, an igniter compound is produced, which is responsible for raising the temperature causing the compound of NaN₃ to break and produce the N2, which inflates the airbag in about 30 seconds.
WHAT HAPPENS TO THE SODIUM THAT IS PRODUCED?
When an airbag deploys, the Na produced is extremely reactive; thus, if there is any moisture around, it will immediately react with it to form NaOH, which is extremely harmful. Thus, certain other chemicals are also included so that the sodium reacts with them to form less harmful compounds.
FAQs
1. What is the chemistry behind an airbag?
The heat produced by accident causes the NaN₃ to break into two products, nitrogen and sodium. 67 liters of N₂ gas is produced from around 130 grams of sodium azide, which is enough to inflate the airbag.
2. Why does an airbag contain KNO₃ and SiO₂?
Sodium metal produced by the breaking down of sodium azide being extremely reactive, KNO₃ and SiO₂ are used to transform the sodium metal into harmless material.
3. Why is nitrogen used in airbags?
Sodium azide is a very stable explosive that explodes at a very high temperature of around 300°C and takes around 40 milliseconds to produce a large volume of nitrogen which is enough to inflate the airbag. Also, N₂ being an inert gas is harmless.
4. Why do airbags always fill evenly?
The main purpose of an airbag is to reduce the speed of the passenger’s progression evenly. Thus, the airbags are evenly inflated.
We hope you enjoyed studying this lesson and learned something cool about the Importance of using an Airbag! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES
- Saved by an Air Bag: https://www.ck12.org/c/chemistry/gas-stoichiometry/rwa/Saved-by-an-Air-Bag/. Accessed 7th March 2022.
- How do Air Bags Works? https://www.scientificamerican.com/article/how-do-air-bags-work/. Accessed 7th March 2022.