Metallic Bond Study Guide
INTRODUCTION
Do solid objects like iron nails and gold jewelry come to mind when you think about metals? If that’s the case, you might be surprised to hear that the gleaming liquid dripping from the beaker in the image below is also a metal. It’s called mercury, and this is the only metal that occurs as a liquid on Earth. What exactly are metals, and what are their characteristics? We will find that out in this article.
The answer to why metals show certain properties lies in the arrangement of electrons in an atom and metallic bonding.
WHAT IS METALLIC BONDING?
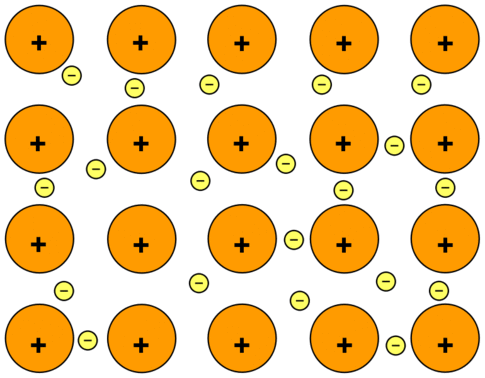
- Metallic bonding is the exchange of free electrons between positively charged metal ions in a lattice.
- Metallic bonds have a substantially different structure than covalent and ionic ones.
- Metallic bonds connect metal atoms, while ionic bonds connect metals to nonmetals, and covalent bonds connect nonmetals to nonmetals.
- The valence electrons from the interacting metal atoms’ s and p orbitals delocalize in metallic bonding.
- That is, rather than circling their individual metal atoms, the interacting metal ions form a “sea” of electrons that encompasses the positively charged atomic nuclei.
- The electrons are then free to travel around between the nucleus.
- This is the reason why this type of bonding is also termed as “electron sea model”.
The peculiarities of metallic bonds explain a variety of distinctive qualities of metals.
PROPERTIES OF METALS
- Since the electrons in the electron sea can move freely and transport electric current, metals are good conductors of electricity.
- Metals are lustrous. Photons just bounce off the metal surface since light cannot permeate it. However, the frequency of light upon which photons are reflected has an upper limit.
- When a force is given to a metal, free-flowing electrons can slide between the immobile cations, preventing them from colliding just like the motion of oil-coated ball bearings gliding past one another. Metals are, as a result, extremely malleable and ductile. They can be shaped by hammering, rolling into thin sheets, or pulling into thin wires.
CONCLUSION:
- The electron sea model is a metallic bonding model in which cations are stationary points inside a mobile “sea” of electrons.
- The peculiarities of metallic bonds explain a variety of distinctive qualities of metals.
FAQs:
1. What is the sea of electrons model?
The electron sea model is a metallic bonding model in which cations are stationary points inside a mobile “sea” of electrons.
2. What does the electron sea model explain?
Many of the physical features of metals are explained by the electron sea model. Since electrons move readily in them, they make good conductors of electricity. Due to the general drifting electrons and the ease with which the cations slide past each other, they are flexible. Because of the unbound electrons, they reflect light.
3. What are mobile electrons?
In metallic bonding, the terminology “mobile electron” is employed. Because the valence electrons in metals are weakly linked and free to travel throughout the metallic lattice, they are referred to as mobile electrons.
We hope you enjoyed studying this lesson and learned something cool about the Sea of Mobile Electrons! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES:
- Bonding in Metals: https://courses.lumenlearning.com/introchem/chapter/bonding-in-metals-the-electron-sea-model/ Accessed 27 Feb 2022.
- Metallic bonding: https://study.com/academy/lesson/metallic-bonding-the-electron-sea-model-why-metals-are-good-electrical-conductors.html Accessed 27 Feb 2022.
- Metallic Bond: https://www.ck12.org/c/chemistry/metallic-bond/lesson/Metallic-Bonding-CHEM/ Accessed 27 Feb 2022.