Proteins Study Guide
Youtube Guide:
Key points:
- Proteins are formed from chains of bonded amino acids, which are formed by DNA.
- There are four stages to protein folding, and each of them has unique properties.
- Enzymes (proteins that catalyze reactions) can be easily denatured if removed from their ideal conditions.
Let’s review concepts on DNA transcription and translation!
- Transcription occurs when DNA is converted into RNA in the nucleus of the cell, which is then translated into amino acids in the ribosomes.
- There are different types of RNA; mRNA (messenger RNA) is used to make proteins, rRNA (ribosomal RNA) is used to make ribosomes, and tRNA (transfer RNA) links the two together.
- Codons, which are nucleotides (Adenine, Thymine, Guanine, Cytosine or Uracil in the case of RNA) in groups of 3, “code” for amino acids.
- Codons, anticodons, start codons, stop codons, introns, and exons are all terms that you should be familiar with.
1. What are the four parts of an amino acid? Explain how amino acids may be grouped according to the physical and chemical properties of the R group.
-
Amino acids are formed from an amino group, carboxyl group, hydrogen, and R group (more on this later) arranged around a central carbon atom
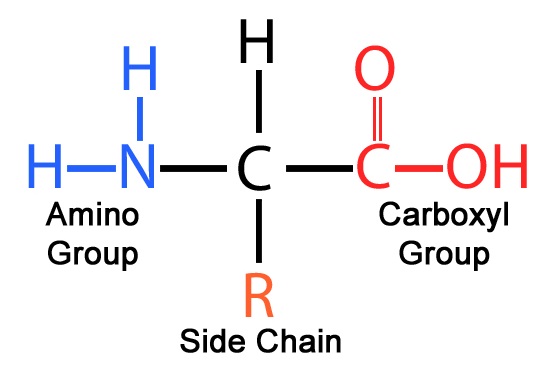 Source
Source -
There are 20 different amino acids, each with a unique R group. Check out this chart for some examples
2. How does a peptide bond form between two amino acids?
-
Amino acids are joined by peptide bonds, which is a dehydration reaction (meaning it produces water).
-
The OH in the carboxyl group of the amino acid on the “left” combines with the H in the amine group of the next amino acid, releasing HOH, or $H_2O$, and hence why it is called a dehydration reaction.
](http://d1j63owfs0b5j3.cloudfront.net/term/images/630-1528466692706.png)
3. What is the difference between a protein and a polypeptide?
- Two amino acids joined by a peptide bond are called a peptide.
- 10+ amino acids (all joined by peptide bonds) are called a polypeptide.
- Proteins are formed by one or more polypeptides folded into a unique 3D structure.
4. What determines protein structure? Why is it important?
- Final structure of the protein is determined by interactions of the different R groups.
- These interactions depend on polarity and change (see this chart for examples)
5. How is the primary structure of a protein determined?
- The primary structure of a protein is simply the linked series of thousands of amino acids in a very long chain.
- Each dot here represents an amino acid, with the lines between them showing the peptide bonds.
- Each protein has a unique order of the 20 amino acids that allows it to function in it’s unique way.
6. What are the two types of secondary protein structures? What is the role of hydrogen bonding in maintaining secondary structure?
- Secondary structure is a series of repeating coiling or folding of the amino acids due to hydrogen bonds between the different amino acids.
- This can form one of two shapes, an alpha helix with regular hydrogen bonds between the amino acids or a beta pleated sheet, which has a backbone bent into repeating zig-zags.
- The organization of the alpha helices and beta pleated sheets (proteins can have both types) in the structure makes up the secondary structure of the protein.
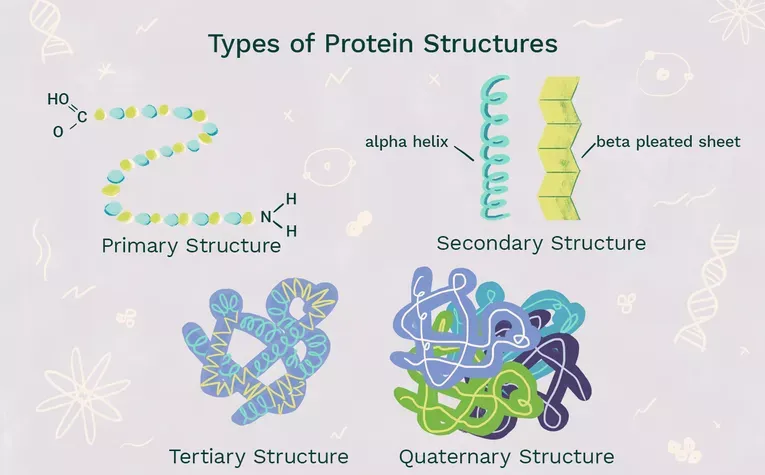
7. How do weak interactions and disulfide bridges contribute to tertiary protein structure?
- Proteins fold into a unique 3D shape in the tertiary structure, based on chemical relationships of the different R groups in the amino acids
- Hydrophobic (water repelling) amino acids fold into the centre of the protein
- Hydrophilic (water loving) amino acids move to the outside of the protein
- Acidic and basic amino acids form salt bridges, stabilizing the protein
- Cysteines sometimes form disulfide bridges, also stabilizing the protein
8. What is quaternary protein structure? How does this structure impact function?
- Two or more tertiary structure combine to form the quaternary structure of a protein
- The final shape here is critical to the proteins function: one wrong amino acid (which can come from a DNA mutation) changes how the protein folds, and thus what it does, if it can still do anything at all. All enzymes, which are critical to proper bodily function, are proteins
9. What are three conditions under which enzymes may be denatured?
- A protein denatures when it unfolds and loses its shape
- This can result from a change in environmental factors such as temperature, salinity, pH, introduction of a foreign chemical or molecule, among others
10. How do chaperonins assist in the proper folding of proteins?
- Chaperonins are small molecules that assist in the folding of proteins
- Nobody quite knows how they work, but they can identify unfolded or misfolded proteins and make them fold again
We hope you enjoyed studying this lesson and learned something cool about Proteins! Join our Discord community to get any questions you may have answered and to engage with other students just like you! We promise, it makes studying much more fun 😎
Sources:
Reece, J. B., & Campbell, N. A. (2011). Campbell biology. Boston: Benjamin Cummings / Pearson.
]]>
