Solubility Study Guide
INTRODUCTION
Did you know that water bodies like rivers, groundwater, and hot springs have a high amount of mineral deposits? Some of the minerals are harmful, and some are useful, but excess amounts of all minerals are harmful to our bodies.
Earlier, having high mineral content in the water was considered therapeutic and good for health. But, through various experiments, it is observed that if the body is unable to dissolve the high mineral content, then it is not good for the body.
WHAT DO YOU MEAN BY SOLUBLE MINERAL?
Soluble minerals are those minerals that can be dissolved in a solvent and form mineral salts. The solvent is mainly water or other aqueous solutions. Many minerals are not soluble in water but are soluble in other mediums. The minerals which form their salts after dissolving in solution form different mineral ions.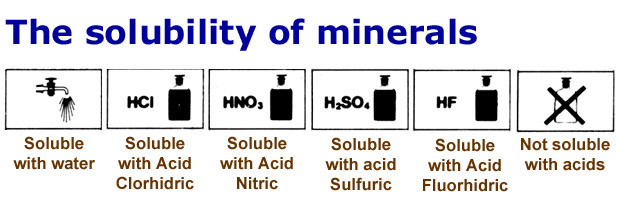 Source
Source
SOLUBLE SALTS DEFINITION
Minerals that dissolve in water form salts. The soluble salts are ionic compounds that dissociate their components during their exchange with a solvent such that it creates a solution with a concentration of at least 0.1 moles per liter at room temperature. They arise from acidic reactions.
WHEN DOES SOLUBILITY DEPEND ON pH?
For ionic compounds comprising basic anions, solubility increases as the pH of the solution is decreased. For ionic compounds that contain anions of negligible or insignificant basicity, such as the conjugate bases of strong acids, solubility is unaffected by changes in pH.
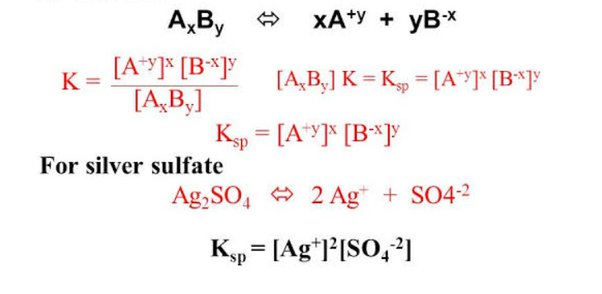
FORMULA AND EXAMPLE PROBLEM
The formula of solubility
Kₛₚ = [A+]ᵃ [B-]ᵇ
where,
Kₛₚ = Solubility product constant A+ = Cation in an aqueous solution B- = Anion in aqueous solutionWrite the solubility product constant expression for equilibrium in a saturated solution of iron(III) hydroxide.
Fe(OH)₃ (s) → Fe₃ (aq) + 3 OH–(aq)
Solution:
Solids do not contribute to mass action expressions, so the solubility product constant is
Kₛₚ = [Fe³]ₑ[OH–]ₑ³
FAQs:
1. Are minerals soluble in water?
Water is a solvent, and it has the ability to dissolve minerals from the rocks with which it comes in touch. The most common dissolved mineral substances are calcium, magnesium, sodium, chloride, potassium, sulfate, and bicarbonate.
2. Are minerals classified by solubility?
No, minerals are classified based on their crystal form or chemical compositions.
3. What are the 3 types of solubility?
On the basis of the concentration of solute dissolved in the solvent, the solubility can be classified into three types:
- Highly Soluble
- Sparingly Soluble
- Insoluble
4. What is the meaning of soluble minerals?
Soluble minerals are those minerals that can be dissolved in a solvent and form mineral salts. The solvent is mainly water or other aqueous solutions.
We hope you enjoyed studying this lesson and learned something cool about Solubility! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES:
- Hot Springs and Mineral Waters: https://www.ck12.org/c/chemistry/solubility/rwa/Hot-Springs-and-Mineral-Waters/. Accessed 7th March 2022.
- Acid-Base Equilibrium: https://www.chm.uri.edu/weuler/chm112/oldchap16answers.html. Accessed 7th March 2022.