Structure of Water Study Guide
INTRODUCTION
Water is one of the most precious resources on the earth. Though 71% of our planet’s surface is covered by water, only 3% is freshwater, and of that 3%, only 1.2% is safe for human consumption. Water is necessary for a multitude of life-sustaining activities, such as drinking and household needs, recreation, agriculture, industry, and thermoelectricity. Its unusual properties make it integral not only for human activities, but also the biological processes that keep every other living being on the planet alive. Let’s learn about its structure to gain a better understanding of why it’s so important.
WHAT IS THE STRUCTURE OF WATER?
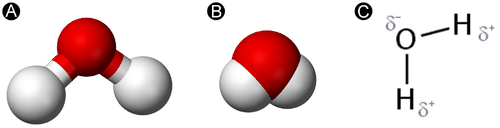
A water molecule consists of one oxygen atom bound to two hydrogens by covalent bonds. A covalent bond is characterized by the sharing of one or more electron pairs between two atoms. When a covalent bond is formed between atoms of significantly different electronegativities, it is classified as a polar bond. Because oxygen has a higher electronegativity than hydrogen, water is considered a polar molecule.
Polar bonds result in an unequal sharing of electrons. The oxygen atom in a water molecule attracts the shared electron pairs to a greater degree and thus acquires a partial negative charge (δ−), while each hydrogen acquires a partial positive charge (δ+). As a result, the H-O-H bond is bent at a 105o angle.
Polar water molecules attract each other by dipole-dipole forces, where the positive end of a molecule is attracted to the negative end of another. Each hydrogen atom on a molecule strongly attracts a lone pair of electrons on an adjacent water molecule. This results in the formation of a hydrogen-hydrogen bond, which is stronger than a dipolar one.
CONCLUSION
- The chemical formula of water is H₂O.
- In a water molecule, two atoms of hydrogen and one atom of oxygen are bound together by covalent bonds.
- The shared electrons of each covalent bond are attracted to the oxygen atom to a greater degree because of its stronger electronegative force.
- The tilt of the shared electron results in hydrogen acquiring a positive charge and oxygen acquiring a partial negative charge.
- The partial charges result in a bent shape of the molecule, arranged at a 105° angle. This is the bond angle of water.
- Polar water molecules attract each other by dipole-dipole forces, where one end of the positive molecule is attracted by the negative end of another polar molecule.
- Each hydrogen atom on a molecule strongly attracts a lone pair of electrons on an adjacent water molecule. This results in the formation of a hydrogen-hydrogen bond.
FAQs
1. What kind of bond joins hydrogen to oxygen in a water molecule?
A polar covalent bond.
2. Does water have a bond angle of 180°?
No, a Water molecule is a bond arranged at an angle of 105°.
3. What is the bond shape of water?
The bond shape of the water is a bent structure.
We hope you enjoyed studying this lesson and learned something cool about the Structure Of Water! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise, it makes studying much more fun! 😎
SOURCES
- Structure of water: https://www.ck12.org/c/chemistry/structure-of-water/lesson/Structure-of-Water-CHEM/. Accessed 8th March 2022.
- Structure of Water: https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/15%3A_Water/15.01%3A_Structure_of_Water. Accessed 8th March 2022.