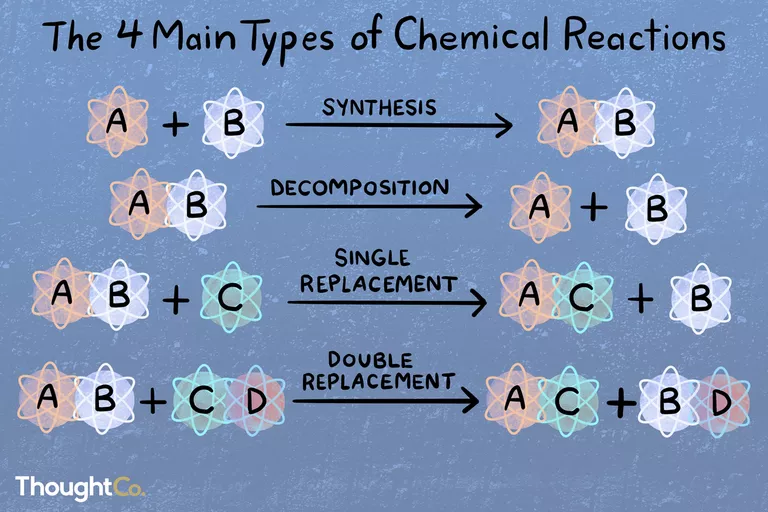
Balancing Single Replacement Reactions Study Guide
INTRODUCTION
Replacements are used in several chemical processes. In compounds, more reactive components replace less reactive ones, and replacement reactions are the name for these reactions.
REPLACEMENT REACTION
When elements in a compound swap locations, this is called a replacement reaction. Ions (electrically charged counterparts of atoms) or ionic compounds are involved in this sort of reaction.
In most cases, a more reactive component substitutes a less reactive element, and the less reactive component is liberated from the combination.
SINGLE REPLACEMENT REACTION
A single replacement reaction happens whenever one element replaces the other in a single component. The general expression for this sort of reaction is:
A + BC → B + AC When potassium combines with water, it is an example of a single substitution reaction. Potassium hydroxide, a white solid chemical, is formed, and hydrogen gas is released.
2K + 2H₂O → 2KOH + H₂ According to the expression, a potassium ion substitutes one of the hydrogen atoms within every water molecule in this process. Because potassium is a powerful oxidizing group 1 alkali metal, it reacts violently with water.
HOW TO BALANCE A SINGLE REPLACEMENT REACTION:
A single replacement reaction’s expression is balanced similarly to any other equation. Coefficients are placed in the front of chemical formulations to ensure that the number of atoms in each element is the same across all sides.
Let’s try and understand this with an example:
-
We put a multiplier of 2 in front of NaCl because there are 2 Cl atoms upon that left side of the equation and another on the right.
-
We now also have two Na atoms here on the right-hand side, but only one is on the left, so we put two next to the Na.
Na(s) + ZnCl₂(aq) → 2NaCl(aq) + Zn(s) -
The equation is now in equilibrium. On both sides of the equation, there seems to be an equal amount of each element.
CONCLUSION:
- In a single replacement reaction, a single element in a compound is replaced by the other.
- Single-replacement interactions can be predicted using the periodic table or a reactivity sequence.
FAQs:
1. How do you balance a single replacement reaction?
A single replacement reaction’s formula is balanced in the very same manner as any other equation. Coefficients are placed in front of chemical formulations to ensure that the number of atoms in each element is the same on both ends.
2. How do you balance an unbalanced reaction?
(i). Count all atom types in the reactants and products.
(ii). To increase the number of atoms or molecules in the components, use coefficients in front of every symbol or formula as needed.
(iii). Steps (i) and (ii) should be repeated until the formula is balanced.
We hope you enjoyed studying this lesson and learned something cool about how to balance single replacement reactions! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES:
- Single-Replacement Reactions. https://www.ck12.org/c/chemistry/single-replacement-reactions/lecture/How-To-Balance-Single-Replacement-Reactions/. Accessed 18 February 2022.
- Single Replacement Reactions. https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_Introductory_Chemistry_(CK-12)/11%3A_Chemical_Reactions/11.07%3A_Single_Replacement_Reactions Accessed 18 February 2022.
- Single Replacement Reactions. https://www.khanacademy.org/science/chemistry/chemical-reactions-stoichiome/types-of-chemical-reactions/a/single-replacement-reactions. Accessed 18 February 2022.
- How do you balance a single replacement reaction? https://socratic.org/questions/how-do-you-balance-a-single-replacement-reaction. Accessed 18 February 2022.