Binary Ionic Compounds Study Guide
The bond formation between two atoms helps to increase the interconnectivity of two atoms to form one compound. If you’ve observed a stenographer in court, they always use code letters to write words faster. Similarly, the abbreviation or symbols of binary ionic chemical formulas are used to refer to a large compound. Let us learn more about binary ionic compounds and how to name them!
WHAT IS THE BINARY IONIC COMPOUND?
The binary ionic formula can be defined as the interrelationship between the ions of one metal and one non-metal. We have seen this in many different bonds like the covalent bond or ionic bond — for instance, metal and non-metal do not form any bonds. But the Binary ionic compounds show the successful formation of metal and non-metal elements bonded as one compound. For example, aluminum nitride ionic compound, potassium iodide compound, etc.
HOW TO WRITE THE BINARY IONIC FORMULA?
It is very simple to write the binary ionic compound formula – if you know the name of the compound, it becomes easier to write down the formula of the compound. The steps you should follow to write the balanced formula of binary ionic bonds are:
- Step 1: You need to first write the signs of the compounds with their ionic charge, like Al₃ and F- in aluminum and fluorine ionic compounds.
- Step 2: See the charges of the signs present. Like in aluminum and fluorine ionic compounds, AI contains 3+ charge, and F contains -1 charge.
- Step 3: Use the crisscross method! Here, the numerical value of each of the ionic charges of the compounds gets crisscrossed, and the compound is formed. Hence, the charges of the elements balance out. This is how AlF₃ is formed.
- Step 4: If the ions are of the same charges, then the magnitudes become equal. For example, the formula for aluminum and nitrogen Al₃ and N₃- becomes AlN.
CRISS-CROSS METHOD
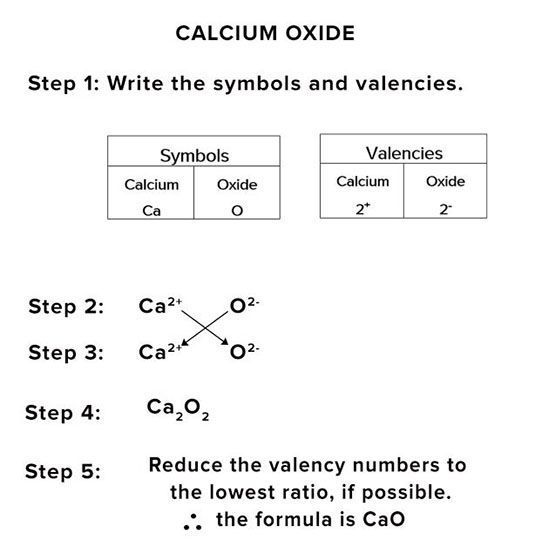
In the crisscross method, the charges are first written in the suffix of the elements, then arrows are used, and the charge on the suffix of the metal element goes to the prefix of the non-metal element, and the charge of the non-metal element will go to the suffix of the metal elements. As shown in the above picture, we can see that aluminum has 3+ charges and chlorine has a -1 charge. The symbols are written with their charges, and then the crisscross method is applied/ so the final compound we get is AlCl₃.
RULES YOU SHOULD FOLLOW TO WRITE THE NAME OF THE BINARY IONIC FORMULA
The rules for writing the name of the Binary ionic formula are as follows:
Rule 1. The name of the cation is written foremost; the anion is written second in the name.
Rule 2. The name of the cation is the exact same as the (neutral) element from which it is derived (e.g., K = “potassium”).
Rule 3. The name of the anion is done by putting the suffix -ide to the tail of the element name (e.g., I- = “iodide”).
FAQs
1. Is aluminum nitride an ionic or covalent compound?
Aluminum nitride, Al-N forms a partial covalent bond. It shows not only covalent nature but also ionic characteristics.
2. Write some examples of the binary ionic compound.
Some examples of binary ionic compounds are iron(III) iodide, sodium iodide, potassium fluoride, etc.
3. What is the aluminum nitride formula?
Aluminum nitride or azanylidynealumane formula is: AlN
We hope you enjoyed studying this lesson and learned something cool about Binary Ionic Compounds: Structure and Examples! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise, it makes studying much more fun! 😎
]]>