Binary Molecular Compounds: Naming and Formulas Study Guide
INTRODUCTION
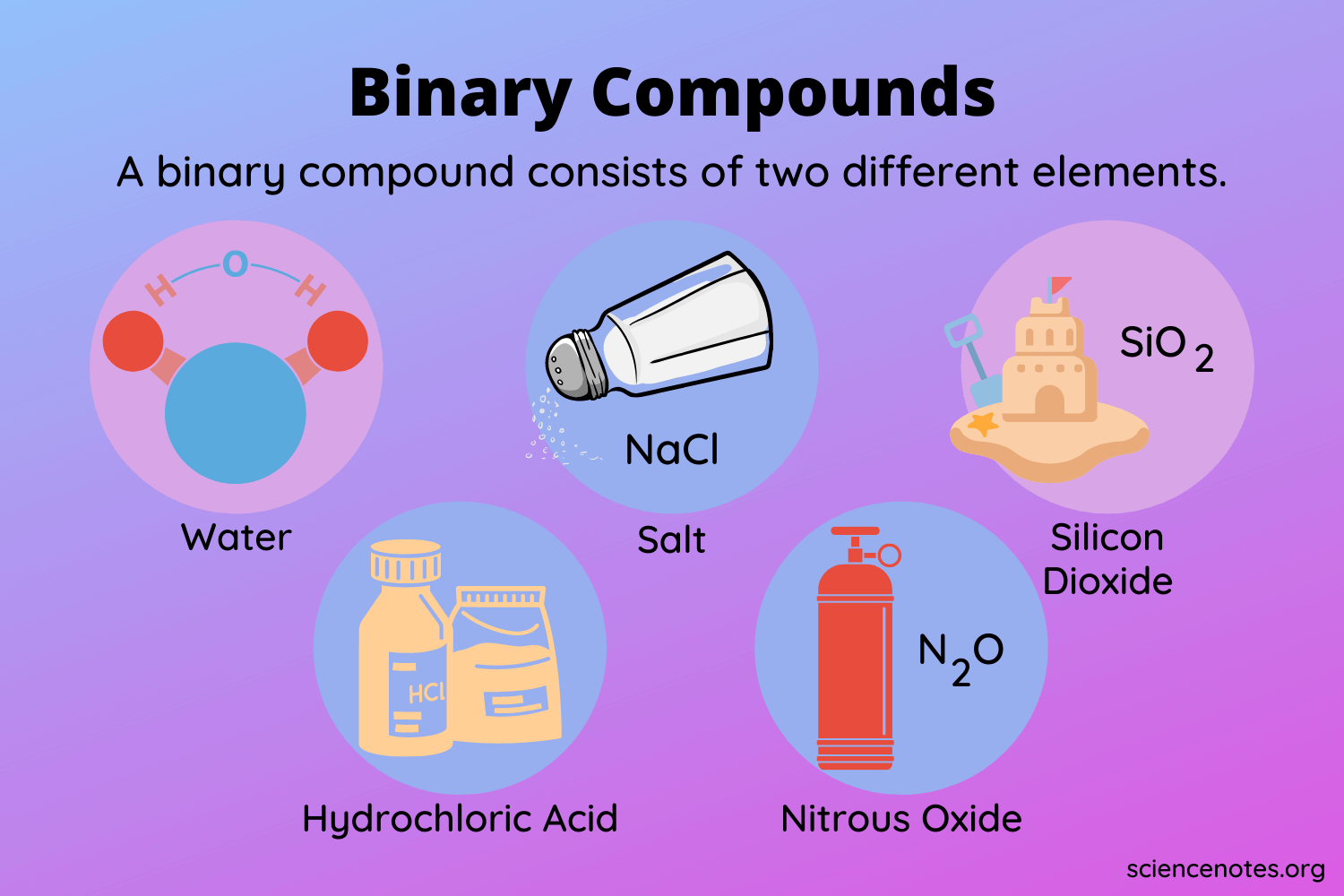
Look at the items below. Do you find anything familiar between these compounds? All these compounds are made up of two different types of elements. Such compounds are known as binary compounds. A binary molecular compound comprises two non-metal atoms that are covalently bound together by sharing electrons.
WHAT ARE BINARY MOLECULAR COMPOUNDS?
- The term “binary” refers to a substance made up of only two types of materials. The sharing of electrons amongst non-metal atoms is referred to as molecular. Covalent bonding is the sharing of electrons. A compound is a chemical that consists of at least two bound atoms.
- When we combine all of those phrases, we get a description: a binary molecular compound is composed of two different types of non-metallic atoms covalently bound together by electron sharing.
- Carbon dioxide, for example, is made up of one carbon atom and two oxygen atoms. Carbon and oxygen are the only elements that are present. These are both non-metallic elements.
NAMING BINARY MOLECULAR COMPOUNDS
Step 1: When both elements in a compound belong to the same period in the periodic table, the element with the lower group number is mentioned first.
Step 2: If the elements belong to two separate periods, the element from the longer time will appear first. The elements should be listed in the correct order in the correct formulations.
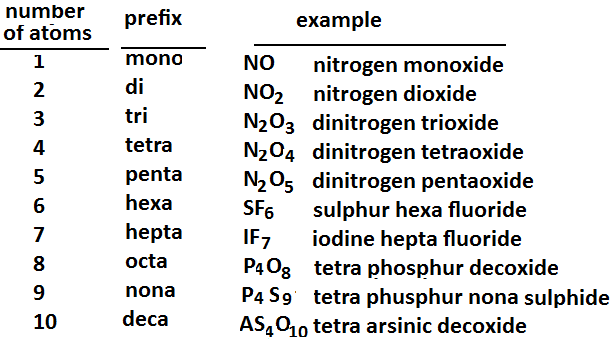
Step 3: Greek prefixes indicate the number of every element in the combination. They’re just placed in front of the element’s name they are changing.
Step 4: The second element’s suffix is removed and replaced with “-ide”
EXAMPLES OF BINARY MOLECULAR COMPOUNDS
CCl₄ = Carbon tetrachloride
N₂O₄ = Dinitrogen tetraoxide
BBr₃ = Boron tribromide
P₃I = Triphosphorus mono iodide
NO = Nitrogen monoxide
N₂O = Dinitrogen monoxide
S₂Cl₂ = Disulfur dichloride
Cl₂O₇ = Dichlorine heptoxide
CONCLUSION:
- A binary molecular compound is composed of two different types of non-metallic atoms that are covalently bound together by electron sharing.
- In binary compound formulations, non-metals are listed in the following order: C, P, N, S, H, Cl, O, F, Br, F.
FAQs:
1. How do you know if a molecule is binary?
To identify a compound, you must first determine what sort of compound it is by looking at its formula. Metal will always be the first element in the formula for a binary ionic compound, whereas a non-metal will always be the second.
2. What is binary in chemistry?
As a reaction between two non-metals, binary molecular compounds are created. Despite the absence of ions, these compounds are termed in the same way as binary ionic compounds.
3. Is H₂O a binary molecule?
H₂O is an example of binary compounds. Binary compounds are exactly two components; neither more nor less.
We hope you enjoyed studying this lesson and learned something cool about Binary Molecular Compounds! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES
- Binary Molecular Compounds: https://study.com/learn/lesson/binary-molecular-compounds-formula-list-prefixes.html Accessed 26 Feb 2022
- Binary Molecular Covalent Compounds: https://www.britannica.com/science/chemical-compound/Binary-molecular-covalent-compounds Accessed 26 Feb 2022
- Binary Molecular Compounds: https://www.ck12.org/c/chemistry/binary-molecular-compounds-naming-and-formulas/lesson/Naming-Binary-Molecular-Compounds-CHEM/?referrer=concept_details Accessed 26 Feb 2022