Biochemical Reactions Study Guide
Introduction
Felix Hoppe-Seyler used the word biochemie in German to describe physiological chemistry in 1877. However, the German scientist Carl Neuberg is frequently credited for coining the term in 1903. In this post, we shall delve deeper into understanding biochemical reactions and their process.
What is a Biochemical Reaction?
- Inside a cell, a biochemical reaction is the transformation of one molecule into another. Under the conditions found in cells, most chemical processes within animals would be impossible.
- For example, most organisms have too low a body temperature for reactions to occur rapidly enough to carry out life activities.
- It’s also possible that reactants are present in such low quantities that they won’t meet and collide. As a result, most biological processes require the use of a catalyst to speed up the process. A catalyst is a substance that accelerates the rate of chemical processes.
What is a Catalyst?
Catalysts are known as enzymes in living organisms. Enzymes are essentially biological catalysts, that may change the pace and specificity of chemical processes inside cells, and mediate biochemical reactions.
What are the different Types of Biochemical Reactions?
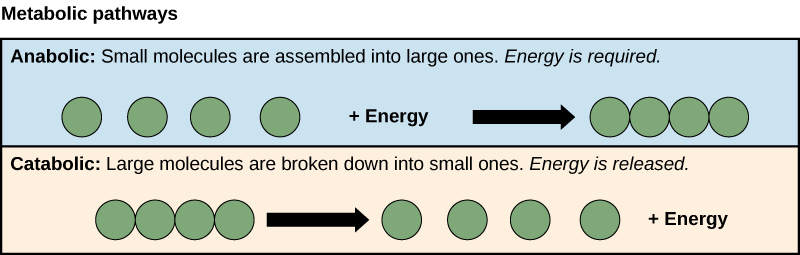
There are two basic types of biochemical reactions- anabolic and catabolic. Sometimes a third type is also defined, which is known as amphibolic pathways.
Catabolic reactions are exothermic processes in organisms.
- These reactions break down molecules into smaller pieces, releasing energy in the process.
- The breakdown of glucose is an example of a catabolic reaction, which releases energy that cells require to carry out life functions.
Anabolic processes are endothermic reactions in organisms.
- These processes combine tiny molecules to form larger compounds.
- The combining of amino acids to make a protein is an example of an anabolic process.
- The respiratory route is an amphibolic pathway because it connects the synthesis (anabolic) and breakdown (catabolic) processes.
Factors in Biochemical reactions:
Regulation
-
Cells and organisms must always maintain a dynamic steady-state to stay alive. This indicates that the preceding reaction delivers the substrate at the same rate as it is transformed to a product for each metabolic reaction in a pathway at the molecular level. The substrate S concentration remains constant even if the rate of metabolite or flux is changed (either raised or lowered). This is referred to as a steady state.
-
External changes might cause a temporary disturbance in the steady state. Each route has its regulatory mechanism for maintaining the dynamic steady-state and homeostasis.
-
To put it another way, biochemical pathways interact in a complicated way for proper regulation to occur. According to the cell’s immediate demands and overall functions, reactions are turned on and off or sped up and slowed down.
-
To maintain homeostatic conditions, living systems have two sophisticated methods for controlling cell metabolism. The first is enzymatic control, whereas the second is hormonal control.
Enzymatic control
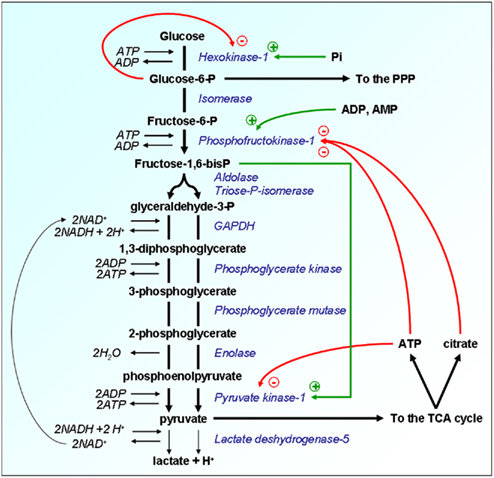
Glycolysis regulation – Glycolysis can be controlled in three ways:
- Hexokinase is a product inhibitor inhibited by glucose-6-P.
- Citrate and ATP (Adenosine triphosphate) inhibit phosphofructokinase.
- ATP, acetyl-Co (Acetyl coenzyme A), alanine, and free fatty acids inhibit Pyruvate kinase.
Pyruvate carboxylase regulates gluconeogenesis through regulating flow.
- It is triggered by acetyl-CoA, indicating that there are enough TCA cycle intermediates and hence a lower demand for glucose.
Role of Enzymes in Biochemical Reactions:
-
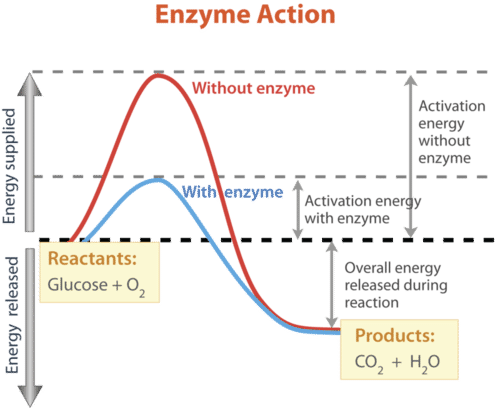
Enzymes are highly effective in accelerating processes. They can catalyze millions of reactions every second. As a result, there might be a significant variation in the rates of biological processes with and without enzymes.
-
Without an enzyme, a typical biochemical process may take hours or even days to complete under normal cellular circumstances, but it takes less than a second with an enzyme.
- Thus, it is clear that enzymes are the most important key for any biochemical reaction. But as discussed above, regulation of the reactions is also of key importance to maintain homeostasis.
Feedback Mechanisms Regulate Enzymatic Action
-
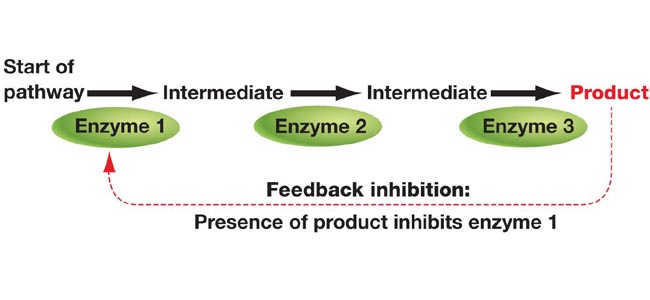
A feedback mechanism is required since it is the only method to prevent wasting energy in producing end-products that are already plentiful.
-
When the final product controls its synthesis rate by inhibiting the initial step, this mechanism is known as feedback inhibition.
-
The term “enzyme inhibition” refers to the process of preventing an enzyme from operating. An inhibitor binds to the active site of an enzyme and prevents the substrate from attaching to itself, halting the metabolic pathway’s sequence. It’s just a conformational shift.
-
The inhibitor merely halts the enzyme action, rendering it inactive, but the binding is only transient. When the inhibitor disengages, the enzyme reverts to its active state and begins to operate on this substrate, reopening the pathways.
Conclusion
- To conclude, a biochemical reaction is the transformation of one molecule into another.
- Most biological processes require the use of a catalyst to speed up the process.
- A catalyst is a substance that accelerates the rate of chemical processes.
- Catalysts are known as enzymes in living organisms.
- Enzymes are essentially biological catalysts. There are two basic types of biochemical reactions- anabolic and catabolic.
- Catabolic reactions are exothermic processes in organisms. These reactions break down molecules into smaller pieces, releasing energy in the process.
- Anabolic processes are endothermic reactions in organisms. These processes combine tiny molecules to form larger compounds.
FAQs:
1. What are the examples of biochemical reactions?
Essentially all the reactions taking place in a living body is a biochemical reaction. This might include photosynthesis, respiration, different digestive reactions, and many more.
2. What are four types of biochemical reactions?
The four major types of biochemical reactions are oxidation-reduction, hydrolysis, condensation, and neutralization.
3. What are the different types of biochemical reactions?
Oxidation-reduction, hydrolysis, condensation, and neutralization.
4. Who is called the father of biochemistry?
Carl Alexander Neuberg is often called the father of biochemistry.
We hope you enjoyed studying this lesson and learned something cool about Biochemical reactions! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun, VR classrooms – we promise, it makes studying much more fun 😎
Sources:
- Biochemical Reactions. https://flexbooks.ck12.org/cbook/ck-12-biology-flexbook-2.0/section/1.14/primary/lesson/biochemical-reactions-bio/. Accessed 25 Nov, 2021.
- Types of Biochemical Reactions. https://bio.libretexts.org/Bookshelves/Introductory_and_General_Biology/Book%3A_Introductory_Biology_(CK-12)/01%3A_Introduction_to_Biology/1.16%3A_Types_of_Biochemical_Reactions. Accessed 25 Nov, 2021.
- 3.10 Chemical Reactions in Living Things. https://humanbiology.pressbooks.tru.ca/chapter/3-10-chemical-reactions-in-living-things/. Accessed 25 Nov, 2021.
- Types of Biochemical Reactions. https://www.cliffsnotes.com/study-guides/biology/biochemistry-i/the-scope-of-biochemistry/types-of-biochemical-reactions. Accessed 25 Nov, 2021.