CBSE Class 10 Science Chapter 1 Revision Notes
Chapter 1: Chemical Reactions And Equations Revision notes
Changes in physical and chemical properties
- Chemical change – the formation of one or more new compounds with different physical and chemical characteristics.
- Physical change – the colour or condition of the substance changes, but no new substance is generated.
Any of the following observations can be used to determine a chemical reaction:
a) A gas’s evolution
b) Thermodynamic shift
d) Precipitation formation
d) A colour change
e) State transition
CHEMICAL REACTIONS
- Chemical reactions are transformations of reactants into products through the formation or breaking of bonds (or both) between distinct atoms.
Equation of words
- A chemical reaction represented in words rather than scientific formulae is known as a word equation. In a chemical reaction, it aids in the identification of the reactants and products.
- As an example, “Sodium interacts with chlorine to generate sodium chloride.
Elements’ symbols and their valencies
- An element’s chemical code is represented by a symbol. Each element has a one- or two-letter atomic symbol that is an abbreviated version of its name.
- An element’s valency is its ability to combine with other elements. It is the number of electrons that an atom loses, gains, or shares as it joins with another atom to create a molecule.
WRITING CHEMICAL EQUATIONS
- A chemical equation is a representation of a chemical process using symbols and chemical formulae for the reactants and products.
- The symbol for solids is “(s)”.
- It’s “(l)” for liquids.
- It’s “(g)” for gases.
- It’s “(aq)” for aqueous solutions.
- “(upward facing arrow)” is used to symbolise the gas created during the reaction.
- “(downward facing arrow)” is used to symbolise the precipitate generated during the reaction.
BALANCING CHEMICAL REACTIONS
- Mass conservation is important.
- Because no atoms may be formed or destroyed in a chemical reaction according to the rule of conservation of mass, the number of atoms for each element in the reactants side must equal the number of atoms in the products side.
- To put it another way, the total mass of the products generated in a chemical reaction is equal to the total mass of the reactants involved.
Balanced Chemical Equations
- A balanced chemical equation is one in which the number of atoms of each element in the reactants side equals the number of atoms in the products side.
- Chemical equations are balanced using the following steps.
- Change the coefficients (the numbers in front of the compound or molecule) while balancing the equation so that the number of atoms of each element is the same on both sides of the chemical equation.
TYPES OF CHEMICAL REACTIONS
Chemical reactions are classified into several groups based on a variety of characteristics.
Here are a few examples:
● Combination
● Decomposition
● Single Displacement
● Double displacement
● Redox
● Endothermic
● Exothermic
● Precipitation
● Neutralisation
Combination reaction Source:
Source:
- Two elements or one element and one compound or two compounds combine to form a single product in a combination reaction.
Decomposition reaction
- When heat, light, or electricity is applied to a single reactant, it decomposes into two or more products.
Displacement reaction
- A less reactive element is displaced from its compound or solution by a more reactive element.
Double displacement reaction
- To produce new products, ions are exchanged between the reactants.
Precipitation reaction
- When two solutions containing soluble salts are mixed, an insoluble substance called precipitate occurs.
Redox reaction
- Oxidation and reduction happen at the same time.
- Oxidation is the process through which a substance loses electrons, receives oxygen, or loses hydrogen.
- Reduction is the process of a substance gaining electrons, losing oxygen, or gaining hydrogen.
- A compound that oxidises another material and then reduces itself is known as an oxidising agent.
- A reducing agent is a chemical that oxidises itself after reducing another molecule.
Endothermic and Exothermic reactions
- An exothermic reaction is one in which heat is released as a result of the reaction. Exothermic reactions characterise the majority of combination reactions.
Al2O3 + Fe + heat Al2O3 + Fe + heat
CO2 + 2H2O + heat = CH4 + 2O2
The reaction is endothermic, which means it requires heat to complete.
C6H12O6 + 6O2 = 6CO2 + 6H2O + Sunlight
The majority of breakdown processes are endothermic in nature.
Corrosion
- Degradation of a substance, generally a metal, over time due to the action of moisture, air, or chemicals in the environment.
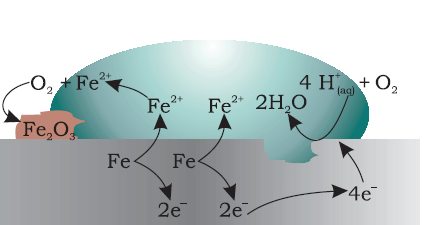 Source:
Source:
2Fe2O3.xH2O = 4Fe(s) + 3O2(from air) + xH2O(moisture) (rust)
Copper corrosion is a type of corrosion that occurs when copper is exposed to air.
CuCO3.Cu(OH)2 = Cu(s) + H2O(moisture) + CO2(from air) (green)
Silver corrosion is a term used to describe the process of a metal corroding.
Ag(s) + H2S (from the air) = Ag2S(black) + H2S (from the air) (g)
Rancidity
- It is the oxidation of fats and oils in food that has been stored for a lengthy period of time.
- It gives food a terrible odour and an unpleasant flavour. When you eat rancid food, you get a stomach illness.
Prevention:
Containers that are airtight
Nitrogen-filled packaging
Refrigeration
Antioxidants or preservatives are added
]]>