CBSE Class 11 Chemistry Chapter 14 Revision Notes
Chapter 14: Environmental Chemistry Revision Notes
- Environmental Chemistry is a branch of chemistry that deals
- It is the branch of science concerned with environmental chemical changes. It encompasses everything like air, water, soil, and the forest.
Pollution of the Environment
It is the result of unfavourable changes in our environment that have negative consequences for plants, animals, and humans.
Pollutants
Pollutant is a term used to describe a material that creates pollution. Pollutants are substances that are solid, liquid, or gaseous. It can be created by human activities or natural occurrences in larger concentrations.
Troposphere
The troposphere refers to the lowest layer of the atmosphere, where humans and other species reside.
It rises to a height of around 10 kilometres above sea level. It contains air, water vapours, and clouds, among other things.
Poisonous gases, smoke fumes, smog, and other pollutants contribute to pollution in this area.
Stratosphere
It stretches from 10 to 50 kilometres above sea level.
The pollution is caused by ozone and other gaseous chemicals found in this region.
Tropospheric Pollution
Pollution in this area is produced by undesired gaseous particles such as sulphur, nitrogen, and carbon oxides, as well as solid particles such as dust, mist, fumes, and smoke.
OXIDES OF SULPHUR
When sulphur-containing coal is burned, they are created.
It’s also created when volcanoes erupt.
Harmful effects:
(i) It is poisonous to both animals and plants.
(ii) In humans, excessive levels of S02 can induce respiratory disorders such as asthma, bronchitis, and emphysema.
(iii) It irritates the eyes, which results in tears and redness.
OXIDES OF NITROGEN
Nitric oxide (NO) and nitrogen dioxide are the two most common nitrogen oxides (NO2).
Principal Sources:
(i) When lightning strikes, the gases N2 and 02 combine to make NO.
(ii) Gasoline combustion in automobiles, hydrocarbon and coal combustion, and so forth.
Harmful effects:
Although nitric oxide is not hazardous to humans, it is highly unstable and readily converts to nitrogen dioxide, which is poisonous in nature. The following are the effects:
(i) It interacts with Ozone (03) in the atmosphere, causing the density of Ozone to drop.
(ii) Higher N02 concentrations harm plant leaves and slow photosynthesis.
(iii) It causes rubber to break.
(iv) Nitrogen dioxide is toxic to a variety of textile fibres and metals.
HYDROCARBONS
Incomplete combustion of fossil fuels in industrial and thermal power plants, as well as automotive exhaust, releases hydrocarbons into the atmosphere on a regular basis, polluting the environment. Negative Consequences:
(i) They cause cancer.
(ii) One of the greenhouse gases is methane.
(iii) They affect plants in a variety of ways, including tissue collapse and leaf shedding.
OXIDES OF CARBON
CO2 is a gas.
By volume, 0.03 percent CO2 is present in the air.
Principal Sources:
(i) By burning of fossil fuels.
(ii) During the manufacturing of cement, limestone decomposes.
(ii) Produced by volcanic eruptions.
(iii) Respiration releases carbon dioxide into the atmosphere.
Harmful effects:
Deforestation and the use of fossil fuels raise the amount of CO2, which is the primary cause of global warming.
Carbon Monoxide (CO) is a colourless, odourless gas.
Major Sources:
(i) Released by the automobile exhaust.
(ii) Incomplete combustion of coal, firewood, gasoline, and other fuels
(iii) At high temperatures, the dissociation of CO2.
Harmful effects:
It forms carboxyhaemoglobin with haemoglobin, which is more stable than the oxygen-haemoglobin complex. When its concentration in the blood exceeds 3-4 percent, the blood’s oxygen carrying ability is severely diminished.
GREENHOUSE EFFECT AND GLOBAL WARMING
Effect of the Greenhouse:
Part gases, such as carbon dioxide, methane, ozone, water vapours, and CFCs, can capture some of the heat radiations emitted by the earth or the sun. The impact is known as the greenhouse effect, and these gases are known as greenhouse gases. As a result, global warming occurs.
Global Warming’s Consequences:
(i) It causes polar ice caps to melt and low-lying places throughout the world to flood.
(ii) As the world’s temperature rises, infectious illnesses such as dengue fever, malaria, yellow fever, and sleeping sickness become more common.
Acid Rain
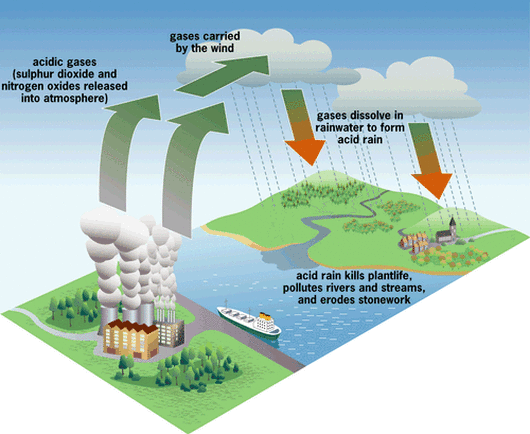 source: Acid rain occurs when the pH of the rainwater falls below 5.6.
source: Acid rain occurs when the pH of the rainwater falls below 5.6.
Due to the solubility of atmospheric carbon dioxide in water, normal rain is somewhat acidic.
Particulate Pollutants
Viable Particulates are microscopic live things that float around in the air. Bacteria, fungus, moulds, algae, and other microorganisms are examples.
Non Viable Particulates:
Smoke: Smoke is a combination of solid and liquid particles created when organic matter is burned.
For example, cigarette smoke and smoke from fossil fuel combustion.
Dust is made up of small solid particles (over 2gm in diameter).
It’s made when solid particles are crushed, ground, and dispersed.
Mist: Mist is formed when liquids such as herbicides and insecticides are sprayed on plants. They fly through the air and condense into mist.
Fumes: Fumes are emitted into the atmosphere by metallurgical processes as well as a variety of chemical reactions.
Particulate Pollutants’ Negative Effects:
I Fine particles less than 5 microns penetrate into the lungs. Such particles can cause significant lung disorders, including lung cancer, if inhaled.
(ii) Larger suspended particles can prevent sunlight from reaching the earth’s surface. This can cause the earth’s temperature to drop and the weather to become cloudy.
POLLUTION
This is the most frequent type of air pollution, and it consists of a mixture of smoke and fog.
There are two forms of smog:
(i) Classical Smog: This type of smog occurs when the weather is cold and humid. It contains sulphur dioxide, smoke, and fog. It’s also known as smog reduction.
(ii) Photochemical Smog: Photochemical smog is caused by sunlight reacting with unsaturated hydrocarbons and nitrogen oxides generated by cars and industry. It is referred to as oxidising smog because it has a high concentration of oxidising chemicals.
Ozone layer depletion
The ozone layer in the high atmosphere acts as a shield, preventing damaging UV rays from reaching the planet.
However, there have been indications in recent years of this layer becoming depleted due to the presence of specific substances in the stratosphere. Depletion is caused by substances such as chlorofluorocarbons (CFCs), nitrogen oxides, chloride, CCl4, and others.
Depletion of the ozone layer has the following effects:
I As a result, several problems such as skin cancer, sunburn, skin ageing, cataracts, and others develop.
(ii) UV rays can destroy a large number of phytoplanktons, reducing fish production.
(iii) It can reduce the moisture content of the soil by promoting surface water evaporation.
(iv) UV rays can harm paints and fibres, causing them to fade more quickly.
Pollution of the water
Water pollution is defined as the presence of undesired components in water that are damaging to humans and plants. The presence of these alien elements can alter the water’s normal qualities.
Water pollution is caused by a variety of factors.
Pathogens: Pathogens are bacteria and other organisms that enter water through sewage and animal waste.
Bacteria such as E. coli and Streptococcus faecalis can be found in human excreta. It is linked to gastrointestinal problems.
Organic wastes, such as leaves, grass, garbage, and other organic debris, can contaminate water.
-
Water pollution is caused by excessive phytoplankton development.
-
By degrading organic waste in water, large populations of bacteria can consume oxygen dissolved in the water.
-
Fish development is reduced when the quantity of dissolved oxygen in water falls below 6 ppm.
-
If too much organic stuff is added to water, the oxygen supply is depleted. This may result in the demise of aquatic life.
Pollutants made out of chemicals
Industrial Wastes: Chemical reactions in industrial units contaminate water to a significant degree. Lead, mercury, nickel, cobalt, and other metals are examples. Because of chemical interactions known as leaching, these chemicals have a negative impact on groundwater and contaminate waterbodies.
Organic substances, such as petroleum products, damage a variety of water sources, such as massive oil spills in seas.
Pesticides include chlorinated hydrocarbons, organophosphates, and metallic salts, among others. They contaminate water by dissolving to a little amount in it. Pesticides are harmful to both plants and animals since they are all poisonous in nature.
PCBS (polychlorinated biphenyls) are chemical substances that are utilised as fluids in transformers and capacitors. These are emitted as vapours into the atmosphere. They poison the water when they mingle with rainwater.
Eutrophication: Eutrophication is the process by which algae-like organisms diminish the amount of dissolved oxygen in water. It is poisonous to aquatic life.
Drinking Water International Standards
Fluoride: Fluoride concentrations up to 1 ppm or 1 mg dm-3 are not toxic to humans.
If it is utilised as drinking water, it will harm living creatures. By converting hydroxyapatite [3Ca3(P04)2- Ca(OH)2] on the surface of the teeth to considerably harder fluorapatite, [3Ca3(P04)2- CaF2], the F ions make the enamel on teeth more tougher. Brown mottling of the teeth occurs when the concentration of F exceeds 2 ppm. Fluoride in excess is also detrimental to bones.
Lead: The upper limit of lead in drinking water is roughly 50 parts per million. Lead may harm the kidneys, lungs, and reproductive system, among other things.
Sulphate: In moderation, it is safe, but in excess, it can be detrimental.
Nitrate: The maximum nitrate level should be 50 parts per million. Drinking water with too much nitrate can induce disorders like methemoglobinemia (blue baby syndrome).
Chemical Oxygen Demand (COD):
To oxidise harmful substances that cannot be oxidised by microbial oxidation, water is treated with K2Cr207 in an acidic media. Back titration with a suitable reducing agent determines the remainder.
The quantity of O2 utilised in the oxidation is determined based on the concentration of K2Cr207 consumed.
Pesticides:
It falls under the following categories:
Insecticides:
Chlorinated hydrocarbons such as DDT, BHC, and others are the most prevalent insecticides.
They stay in the soil for a long time since they are not water soluble. They are absorbed by the soil and infect root crops such as radish, carrot, and other root vegetables.
Herbicides
These are the chemicals that are used to control weeds.
Sodium chlorate (NaCl03) and sodium arsenite (Na3As03) are two typical herbicides, however arsenic compounds are no longer favoured due to their toxicity.
Fungicides
The most prevalent fungicides are organomercury compounds. When it dissociates in the soil, mercury is released, which is very poisonous and detrimental to crops.
]]>