CBSE Class 11 Chemistry Chapter 5 Revision Notes
Chapter 5: States of Matter Revision Notes
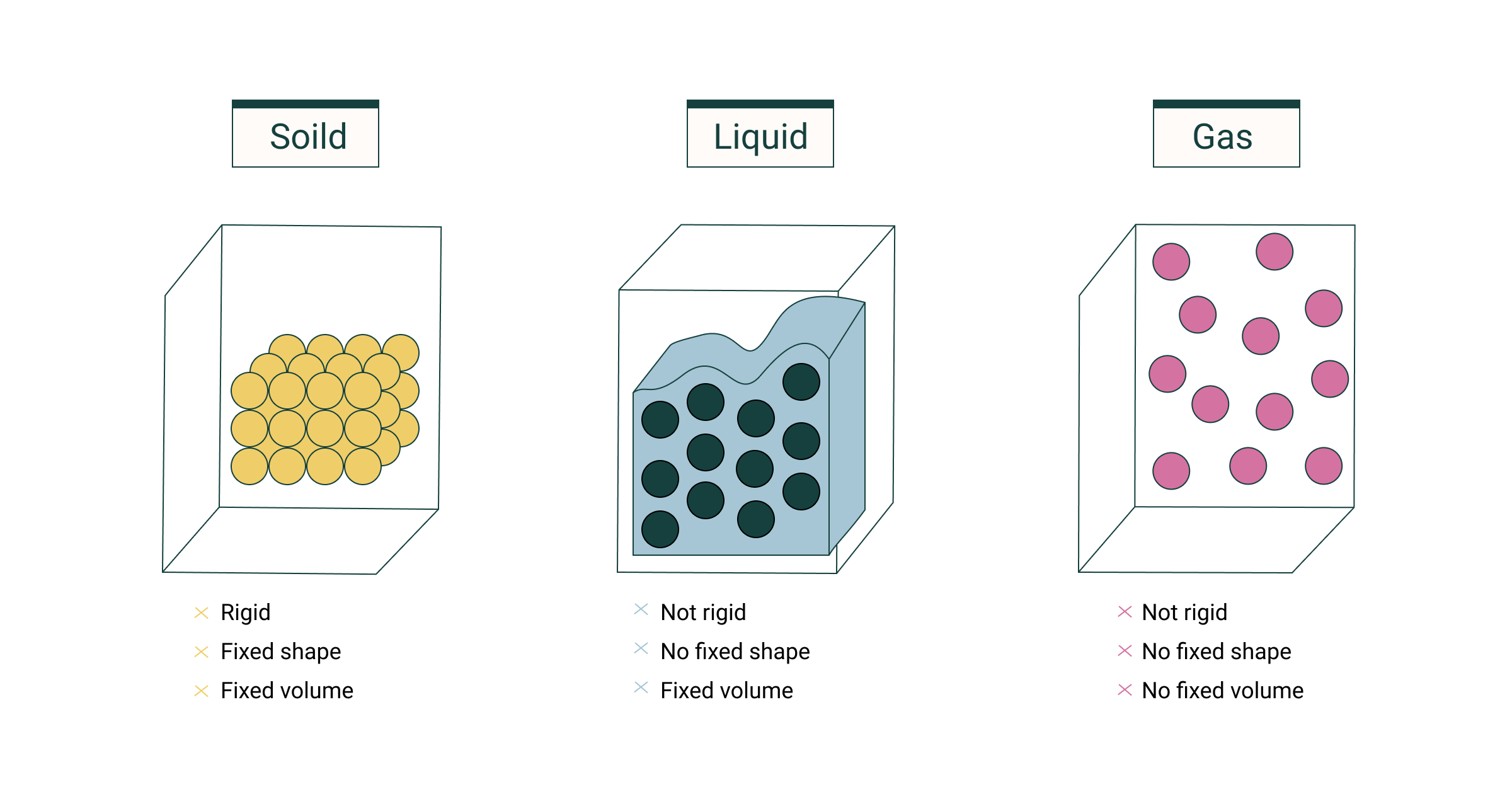
- Matter is defined as everything that takes up space and has a defined mass. Solid, liquid, and gaseous phases are the most common states of matter.
- Solids have a defined form as well as a defined volume. Because the particles in solids are so densely packed, the intermolecular force of attraction is stronger.
- Liquids do not have a defined form, but they do have a defined volume. The intermolecular force of attraction in liquids is less than in solids. As a result, the particles are not in a fixed place.
- Gases do not have a defined shape or volume. Because the particles are so far away, there is no attraction between them.
INTERMOLECULAR FORCES
- The forces of attraction and repulsion between interacting particles are known as intermolecular forces (atoms and molecules).
- Van der Waals forces are attractive intermolecular forces.
- Dispersion forces, also known as London forces, dipole-dipole forces, and dipole-induced dipole forces are examples of these forces.
- Hydrogen bonding is a sort of dipole-dipole interaction that is exceptionally strong.
1) London Forces or Dispersion Forces
- Atoms and non-polar molecules have no dipole moment and are electrically symmetrical.
- However, in an atom, the nucleus is displaced to one side and the electrons to the other at any given time.
- As a result, a temporary dipole (temporarily dipole) is formed.
- For a brief period of time, this leads in the creation of an instantaneous dipole on the nearby atom.
- Atoms with transient dipoles attract each other.
- London forces or dispersion forces are the forces of attraction between transient dipoles. These forces are only significant over short distances.
2) Dipole – Forces of Dipole
- Between molecules with permanent dipoles, dipole-dipole forces act (polar molecules).
- These molecules interact with the molecules around them.
- Because only partial charges are involved, this interaction is stronger than London forces but weaker than ion-ion contact.
- The attractive force reduces as the distance between the dipoles increases. HCl, for example.
3) Dipole Forces Induced by Dipoles
- The polar molecules and the non-polar molecules are attracted to one other by this form of attraction.
- By deforming the electronic cloud of the electrically neutral molecule, the permanent dipole of the polar molecule produces dipole on it.
- As a result, the other molecule develops an induced dipole.
- Dipole – induced dipole force describes the attraction between these molecules.
GAS LAWS
Boyle’s Law
At constant temperature, the volume of a given mass of gas is inversely proportional to its pressure.
Vp = K or V 1 / p
K is a constant whose value is determined by the gas’s mass, temperature, and nature.
Charles’ Law
At constant pressure, the volume of a given amount of gas grows or decreases by 1 / 273 of its volume for each degree of temperature rise or fall.
Vt = Vo (1 + t / 273) t constant p
or
At constant pressure, the volume of a given mass of a gas is exactly proportional to the absolute temperature.
V ∝ T (at constant p), V / T = constant or V1 / T1 = V2 / T2
Absolute zero is the lowest temperature at which the volume of a gas may potentially reach zero. It’s the same as 273.15K or O°C.
Gay Lussac’s Law
For each degree of temperature rise or fall, the pressure of a given amount of gas increases or falls by 1 /273 of its pressure at constant volume.
pt = po (1 + t / 273) at constant V and n
or
The absolute temperature is exactly proportional to the pressure of a given mass of a gas at constant volume.
p ∝ T or p = KT or p / T = K at constant V and n or P1 / T1 = P2 / T2
Avogadro’s Law
It says that under the same temperature and pressure, identical volumes of all gases contain the same number of molecules.
Mathematically
V infi; n (at constant T and p)
Alternatively, V / n = K
Equation for a Perfect Gas
(Boyle’s law) V1 / p, T, and n constants
(Charles’ law) V T, p, and n constants
The constants V n, p, and T (Avogadro’s law)
⇒ V ∝ nT / p
or pV ∝ nT
Alternatively, pV = nRT.
This is referred to as the ideal gas equation. The universal gas constant is R.
Density is derived from the ideal gas equation.
(where M = molecular mass) d = pM / RT
The Kinetic Theory of Gases is a theory that describes how gases move.
The following are the theory’s main assumptions:
-
A gas is made up of many tiny particles known as molecules.
-
When compared to the overall volume of the gas, the amount occupied by gas molecules is minimal.
-
Gas molecules are in a constant state of fast random motion. The molecules clash with one another and with the container’s walls.
-
Because the molecules are ideal elastic bodies, no kinetic energy is lost during collisions.
-
The gaseous molecules have no attraction forces between them.
-
A gas’s pressure is caused by the bombardment of gas molecules against the container’s walls.
-
Different molecules have different velocities, which means they have different energies. The average KE is proportional to absolute temperature in a direct relationship.
Abuse of the Ideal Behaviour
The gases depart significantly from their ideal behaviour under high pressure and low temperature. The compressibility factor (Z) can be used to express the deviation.
pV / nRT = Z
pV = nRT, Z = 1 in the case of an ideal gas
pV nRT, Z 1 in the case of actual gas
Deviation in the negative direction In this scenario. When the value of Z is greater than one, the gas is more compressible.
Deviation in the positive direction In this scenario. Gases with a Z greater than one are less compressible.
The elements that influence the deviation are as follows:
(i) The gas’s nature The most easily liquefiable and highly soluble gases, in general, have a bigger deviation.
(ii) Tension At high pressure, the divergence is greater. At low pressure, CO2 and N2 show a negative deviation, but at high pressure, they show a positive deviation.
(iii) At low temperatures, the deviation is greater, and H2 and He always have positive deviations at O°C.
Gas Liquefaction and Critical Points
- Liquefaction is the process of transforming a gas into a liquid.
- The liquefaction of a gas occurs when the intermolecular forces of attraction in the liquid become too strong. It is possible to liquefy a gas.
(i) increasing the amount of pressure
(ii) lowering the temperature
State of Liquid
- If a substance has a melting point below room temperature and a boiling point above room temperature, it is said to have a low melting point.
- Liquid is the name given to the material.
- Matter has certain properties when it is in a liquid condition. Between solids and gases, structure and molecular mobility exist.
Liquids’ Characteristics
(i) The pressure of vapour The vapour pressure of a liquid is the pressure exerted by vapours above the liquid surface when they are in equilibrium with the liquid at a certain temperature.
A liquid’s vapour pressure is determined by:
(i) Liquid’s nature
(ii) Temperature: As the temperature rises, so does the vapour pressure.
(ii) Boiling point refers to the temperature at which a liquid’s vapour pressure equals atmospheric pressure.
Boiling point at 1 atm pressure is referred to as normal boiling point.
Boiling point at 1 bar is referred to as normal boiling point. The boiling point changes in a linear relationship with the external pressure.
(iii) Surface Tension: It’s the force per unit length operating perpendicular to the imaginary line drawn on the liquid’s surface. (gamma) is the symbol for it.
Nm-1 is a SI unit.
Dimensions: kgs-2
The attractive interactions between molecules determine the amount of a liquid’s surface tension. It is determined using a device known as a stalagmometer.
As the temperature rises, the surface tension drops.
Surface tension causes the liquid to rise or fall in a capillary tube.
(iv) Viscosity is a term used to describe the viscosity of a Viscosity is a measurement of flow resistance caused by friction between fluid layers.
]]>