CBSE Class 11 Chemistry Chapter 9 Revision Notes
Chapter 9: Hydrogen Revision Notes
- The effect of dilute H2O4 on iron was discovered by Henry Cavendish in 1766.
- It was given the term ‘inflammable air’ by Lavoisier (Creek: Hydra = water, genes = creator).
- It can happen in both the free and combined states.
Hydrogen’s Place in the Periodic Table
- Hydrogen has properties that are similar to both alkali metals (group I) and halogens (group 17), yet it also has features that are distinct from each. That is why hydrogen is referred to be a “rogue element.”
- However. It was assigned to group 1 based on its configuration 1s1, which is the basis for contemporary element categorization.
DIHYDROGEN [H2]
Methods of Preparation
(a) Lab production
(i) Acids can be liberated by metals with a lower reduction potential than H.
- Because pure zinc reacts slowly, it is not utilised.
- The creation of electrochemical couples accelerates the rate of reaction when certain contaminants are present.
- Because it oxidises, converting H2 to H2O, cone sulphuric acid is not utilised.
Zn + 2H2SO4(conc.) ZnSO4 + SO2 + 2H2O ZnSO4 + SO2 + 2H2O
(ii) It can also be made by combining zinc with aqueous alkali.
(b) Commercial dihydrogen production
(i) Using acidified water as an electrolyte(ii) From the gaseous state of water (Bosch process)Carbon dioxide is eliminated by dissolving it in water at high pressure (20-25 atm) and collecting the hydrogen that remains.(iii) From steam (Lane's technique) When hydrogen is generated, superheated steam is passed through iron filings heated to around 1023-1073 K.Dihydrogen’s Physical Properties
- Dihydrogen is a flammable gas that is colourless, odourless, and tasteless.
- It is lighter than air and water insoluble.
- Litmus is unaffected by it.
Dihydrogen’s Applications
- It is utilised in the production of CH3OH.
- It generates a temperature of 2850°C, while an oxy-atomic hydrogen flame generates a temperature of 4000°C, hence it is employed in an oxy-hydrogen flame.
- The synthesis of NH3, which is utilised in the production of HNO3 and fertilisers, is the most common usage of H2.
- Rocket fuel is made of liquid hydrogen (LH2).
- In the extraction of metals, H2 is utilised as a reducing agent.
- In a fuel cell, hydrogen is utilised to generate electricity.
- In the production of synthetic gasoline, hydrogen is used.
DIFFERENT FORMS OF HYDROGEN
Atomic Hydrogen
- It has a half-life of 0.33 seconds and is very reactive.
Nascent Hydrogen
- Nascent hydrogen is more reactive than conventional hydrogen because it is freshly created.
- It allows for the reduction of certain compounds that would otherwise be impossible to do with regular hydrogen.
- It is impossible to separate it.
Adsorbed Hydrogen
- Occlusion is the process of hydrogen adsorption on a metal surface.
- Many chemical changes occur as a result of hydrogen, including reduction and hydrogenation.
- As the temperature rises, the occlusion diminishes.
Ortho and Para Hydrogen
- The nuclear spins of a hydrogen molecule are in the same orientation, which is known as ortho hydrogen.
- When the nuclear spins arc in the other direction, on the other hand.
- It’s called para hydrogen. At room temperature, hydrogen is made up of 75% ortho hydrogen and 25% para hydrogen.
HYDRIDES
- Hydrogen compounds with metals and non-metals are known as hydrides.
Ionic Hydrides
- These are made by heating elements from groups I and II (excluding Be and Mg) in hydrogen.
- These are crystalline white colourless solids with high m.p. and b.p. that are quickly dissolved by water, CO2, or SO2.
Molecular or Covalent Hydrides
- These are made up of p-block elements with a greater electronegativity than hydrogen.
**Electron deficient Hydrides **
- These are hydrides that lack the necessary number of electrons to form typical covalent bonds, such as BH3, AlH3, and others.
Electron Precise Hydrides
- These are hydrides that have exactly the right amount of electrons to form typical covalent bonds. Group 14 hydrides, for example (CH4, SiH4, etc.)
Electron rich Hydrides
- These are hydrides with more electrons than are necessary for conventional covalent bonds to form.
- Hydrides of the 15th, 16th, and 17th groups, for example (NH3, PH3 ,H2S, HF, HCl, etc). Lone pairs of electrons make up the extra electrons in these hydrides.
Metallic or Interstitial Hydrides
- Interstitial hydrides are formed when transition metals and rare earth metals mix with hydrogen. They have metallic characteristics and are effective reducers.
- For example, LaH2.76, TiH1.73 are non-stoichiometric hydrides whose composition changes with temperature and pressure.
- The hydride gap is a section of the Periodic Table where metals in groups 7, 8, and 9 do not produce hydrides.
Complex Hydrides and Polymeric Hydrides
- Elements with electronegativity in the range of 1.4 to 2.0, such as (BeH2)n, (AlH3)n, and others, produce polymeric hydrides.
- In complex hydrides, such as LiAlH4, LiBH4, and others, H– functions as a ligand and is connected to the central metal atom.
WATER
- The most plentiful and widely spread substance on the planet is water. It can be found in all three physical states.
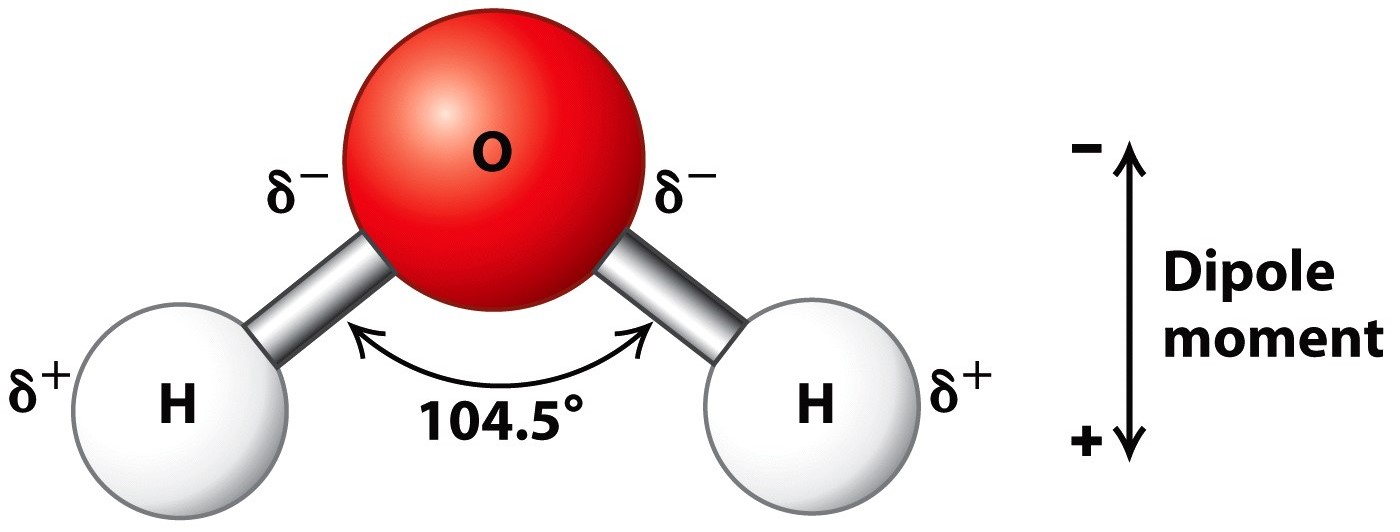
- H2O is a covalent molecule with sp3 hybridised oxygen. It has a curved structure.
Water’s Physical Characteristics
- Water is a tasteless, odourless, and colourless liquid. Due to hydrogen bonding, it possesses exceptionally high b.p., f.p., and heat of vaporisation.
- Because pure water is a poor conductor, it is converted to one by adding a little amount of acid or alkali.
- Ice has a lower density (mass per unit volume) than water and hence floats on top of it.
- At 4°0, the density of water reaches its maximum.
- Water has a high dielectric constant of 78.39, making it a highly polar solvent. It reacts efficiently with polar or ionic compounds, releasing a significant amount of energy as a result of the ion-dipole contact.
- The ability of covalent substances such as urea, glucose, and C2H5OH to form hydrogen bonds with water causes their dissolution.
Water’s Chemical Properties
-
Water is naturally amphoteric.
-
Water interacts with both metals and non-metals in redox processes.
-
Water may exist in five different forms in hydrated salts: coordinated water, hydrogen bonded water, lattice water, clathrate water, and zeolite water.
-
Water hydrolyzes a variety of substances, including calcium hydride, calcium phosphide, and others.
Water Purification
- There are two procedures involved.
- Impurities suspended in the air are removed.
- Getting rid of the germs.
- Suspended particles are removed using alum coagulation and filtering.
- Bacteria are killed by exposing them to sunlight, boiling, chlorination (treatment with liquid Cl2 or bleaching powder), ozonisation, and the addition of CuSO5.
Soft and Hard Water
- Water that creates a lot of lather with soap is called soft water, whereas water that develops scum with soap is called hard water.
Types of Hardness of Water
- Temporary abrasion It is caused by the presence of calcium and magnesium bicarbonates.
- The presence of calcium and magnesium chlorides and sulphates causes permanent hardness.
Removal of Temporary Hardness
It is possible to achieve:
(a) Soluble bicarbonates are transformed to insoluble carbonates by boiling.
(b) Using Clark’s method By using lime water or lime milk.
Permanent Hardness Removal
(i) Adding washing soda to the mix Carbonates are formed when calcium or magnesium salts precipitate.
(ii) Adding caustic soda to the mix Caustic soda can be used to eliminate both temporary and permanent hardness.
(iii) Calcium and magnesium phosphates are precipitated by adding sodium phosphate (Na3PO4).
Magnesium, in the form of magnesium phosphate, Mg3(PO4)2, similarly precipitates out.
(iv) Calgon’s method Calgon is sodium hexametaphosphate (Na6P6O18). When this Calgon is mixed with hard water, it forms a soluble complex.
Similarly. Water becomes free of Ca2+ and Mg2+ ions when Mg2+ precipitates as Na2[Mg2(PO3)6].
(v) The permutit procedure Permutit (Na2Al2Si2O8.xH2O) is a hydrated sodium aluminium silicate. It does this by exchanging sodium ions for the divalent ions Ca2+ and Mg2+.
When Permutit is completely depleted, it can be regenerated by treating it with a 10% sodium chloride solution. It is the most effective way for gelling water with no hardness.
(vi) By synthetic resins
There are two kinds of these:
(a) Sulphonic acid-containing cation exchange resins are large molecules (-SO3H). It is initially converted to sodium salt using the formula RNa. Ca2+ and M2+ are exchanged and eliminated when hard water passes through it.
A solution of NaCl can be used to regenerate resins like permutit.
(b) Anion exchange resins, on the other hand, are large molecules that can exchange anions. They have an amino group in them.
Pure distilled water is created after passing the water through cation resins and then anion resin.
The Hardness Scale
- It is a tool for determining the degree of difficulty in a product.
- The number of parts of calcium carbonate or equivalent to other calcium and magnesium salts present in one million parts of water by mass is referred to as the degree of hardness. It is measured in parts per million (ppm).
- Hardness degree (in ppm) = (weight of CaCO3 (g)/ weight of hard water (g) x 106
Hydrogen Peroxide[H2O2]
- In 1818, J.L. Thenard discovered H2O2. It’s a crucial chemical in the treatment of home and industrial effluents for pollution management.
Strength of Hydrogen Peroxide
- The amount (in mL) of oxygen emitted at NTP by decomposition, or 1 mL of that sample of H Z 0 2, is the most typical way to express the strength of H2O2. A ’10 volume’ H2O2 solution really means “1 mL of such an H2O2 solution yields 10 mL of oxygen at NTP when decomposed by heat.”
(i) Strength of H2O2 in terms of normality
(ii) X = 11.2 x molarity (iii) percent strength = (17/56) x volume strength
Hydrogen Peroxide Storage (H2O2)
- It’s kept with traces of alcohol, acetanilide, or sodium pyrophosphate, which slow down the rate of hydrogen peroxide breakdown.