Energy Levels Study Guide
Introduction
- The energy level of an atom is the amount of energy stored in its orbitals.
- Bohr’s atomic model has stated this concept widely and realized that the emitting light from any specific atom is due to its specific frequencies.
- And further to prove his point, he introduced Bohr’s model of the Hydrogen atom which resulted in the energy level example being quantized.
WHAT IS THE ENERGY LEVEL
An atom usually consists of electrons, which revolve around its nucleus in its fixed orbits. While moving all around its orbit, they are not allowed to freely move and change their random position. This is because of their certain energy level.
Therefore, energy levels are the fixed distance of electrons from the nucleus to that particular atom. They are also known as electron shells. K, L, M, and N are the first few-electron shells.
ENERGY STATE
The required energy to excite an electron if provided will cause the electrons to absorb this energy and shift to the higher level of energy from the lower level.
For example: the Neon atom energy levels.
- There are two electrons in energy level I of a neon atom, but the remaining eight electrons are in energy level II, which can only hold eight electrons.
- This indicates that its outermost energy level is full.
- Neon atoms are therefore stable.
- Moreover, while shifting electrons from one shell to another, they emit a certain amount of energy in the form of light.
- And there itself, the emission and absorption take place (the return of electrons to their original energy level is called emission and the gain in energy when electrons jump up to higher energy levels is called absorption).
- These lowest possible electron energy levels are known as the ground state; on the other hand, all the higher energy levels are called the excited state.
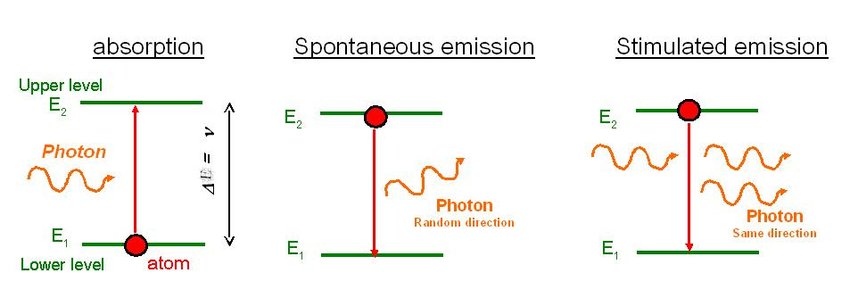
ENERGY LEVEL DIAGRAMS
We can understand the emission of light and absorption at the same wavelength by understanding the electron energy level diagrams.
- It says when an atom or molecule absorbs light or colloid with each other, it produces enough energy, which enables them to move from lower to higher energy levels.
- Further, the emission is initiated due to the excited state of the atom either by collision or absorption. There is no interaction between photons in spontaneous emission, and the direction and phase are random; whereas, stimulated emission occurs when an excited electron interacts with a photon.
SUMMARY
- Every atom is surrounded by different orbits consisting of particular energy, and this energy is known as the energy levels of an atom.
- The electrons present in the orbits shift from higher energy levels to lower or vice versa in the given state.
- The energy level is also known as the electron shell.
- These shells carry certain electrons known as valence electrons.
FAQs
1. What are the 4 energy levels?
If you are wondering how many energy levels are there, there are four energy levels: s, p, d, and f.
2. What is the energy level in physics?
The energy level in physics stands for the set of values of the total energy of a subatomic particle that is confined by a certain amount of force into a confined space.
3. What is the 5 energy level?
Five atomic energy levels are -13.6 eV, -3.4 eV, -1.51 eV, -.85 eV, -.54 eV.
We hope you enjoyed studying this lesson and learned something cool about interpreting Energy Levels In Chemistry! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun!😎
REFERENCES
- Energy Level of an Atom: https://www.vedantu.com/physics/energy-level-of-an-atom. Accessed 28 Feb 2022.
- Energy Level Diagram: https://byjus.com/chemistry/energy-level-diagram/. Accessed 28 Feb 2022.
- Energy Level: https://flexbooks.ck12.org/cbook/ck-12-chemistry-flexbook-2.0/section/5.12/primary/lesson/energy-level-ms-ps/. Accessed 28 Feb 2022.