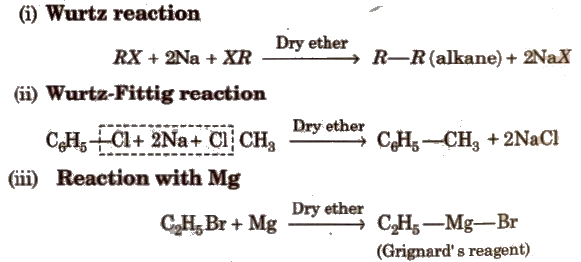CBSE Class 12 Chemistry Chapter 10 Revision Notes
Chapter 10: Haloalkanes and Haloarenes Revision Notes
- According to the type of halogen present, haloalkanes are classified as fluoro, chloro, bromo, or iodo compounds, and as mono-, di-, tri-, tetra-haloalkanes, etc., according to the one, two, three, four, or more halogen atoms present in their molecule.
- Alkyl halides are classified as primary (1°), secondary (2°), or tertiary (3°) based on the halogen atom attached to the primary, secondary, or tertiary carbon atoms.
- The shared pair of electrons is closer to the halogen atom due to the electronegativity difference between carbon and halogen. As a result, the halogen has a slight negative charge and the carbon has a slight positive charge. As a result, the C-X bond is classified as a polar covalent bond.
Preparation of Haloalkane
- Haloalkanes can be made by displacing the alcoholic group in alkyl alcohol with halogen acid, such as PCl5 or PCl3.
- Haloalkanes can also be made by reacting alkene and alkyne with halogen acids or halogens.
- Alkyl halides can also be made by halogenating an alkane with a free radical.
Preparation of Haloarenes
- Side chain halogenation or nuclear halogenation of aromatic hydrocarbons can produce haloarenes.
- Made with diazonium salts: -Through the Sandmeyer reaction and/or through the Gattermann reaction
Chemical Reactions of Haloalkane
(a) Nucleophilic substitution reactions:
- Due to the electron-repelling nature of the alkyl group (-), the C-X bond in alkyl halides is more polar and thus more easily undergoes nucleophilic substitution reaction.
- There are two kinds of these:
(1) SN1 (Nucleophilic substitution, unimolecular): In these reactions, rate = k [RX], i.e., rate is independent of nucleophile concentration and occurs in two steps. Polar solvents are ideal for such reactions.
(2) SN2(Substitution, nucleophilic, bimolecular): In such reactions, rate = k [RX] [Nu]–, i.e., reaction rate is proportional to nucleophile concentration and occurs in a single step.
- In an SN2 reaction, complete stereochemical inversion occurs.
- In an SN1 reaction, racemisation occurs.
(b) Elimination Reaction
- When a haloalkane with a β-halogen atom is heated with an alcoholic solution of potassium hydroxide, a hydrogen atom from the α-carbon atom and a halogen atom from the a-carbon atom are eliminated.
- As a result of the reaction, an alkene is formed. The reaction is also known as β-elimination.
(c) Reactions with Metals
- Due to the below mentioned reasons, aryl halides are extremely resistant to nucleophilic substitution reactions.
(a) In haloarenes, resonance causes the lone pair of electrons on the halogen atom to delocalize on the benzene ring.
(b) The halogen atom in haloalkanes is attached to sp3-hybridized carbon, whereas the halogen atom in haloarenes is attached to sp2– hybridized carbon.
- The electrophilic substitution reactions of the benzene ring, such as halogenation, nitration, sulphonation, and Friedel crafts reactions, are all carried out on haloarenes. The halogen atom is o, p-directing and slightly deactivating.
- Trichloromethane (Chloroform) is primarily used in the manufacture of the freon refrigerant R-22. Chloroform is kept in sealed dark-colored bottles that are completely filled to keep air out. Carbonyl chloride is a highly poisonous gas that is slowly oxidized by air in the presence of light (Phosgene).
- Freons are methane and ethane chlorofluorocarbon compounds. They are nontoxic, non corrosive, and easily liquefiable gasses that are extremely stable and unreactive.
- Freon-12 (CCl2F2) is the most commonly used freon in industry.