CBSE Class 12 Chemistry Chapter 11 Revision Notes
Chapter 11: Alcohols, Phenols and Ethers Revision Notes
- Alcohols and phenols are classified as monohydric, dihydric, trihydric, or polyhydric depending on how many hydroxyl groups they have in their molecules.
- Alcohols with the OH group attached to a primary, secondary, or tertiary carbon atom are referred to as primary (1°), secondary (2°), and tertiary (3°) alcohols, respectively.
- If the alkyl or aryl groups attached to the oxygen atom are the same, ethers are classified as simple or symmetrical, and if the two groups are different, ethers are classified as mixed or unsymmetrical.
Alcohols Preparation
(a) From Alkenes
- Acid-catalyzed hydration: According to Markovnikov’s rule, the addition reaction takes place.
- By hydroboration-oxidation
(b) From carbonyl compounds
- By reducing aldehydes and ketones: Aldehydes produce 1° alcohols, while ketones produce 2° alcohols.
- By reduction of carboxylic acids and esters.
- From Grignard Reagents
Phenol Preparation
-
By halogen substitution in
(a) Haloarenes (b) Sulfonic acid group: In benzene sulphonic acid can be used to make phenols.(c) Diazonium salts are hydrolyzed(d) Cumene as a source of industrial materials:
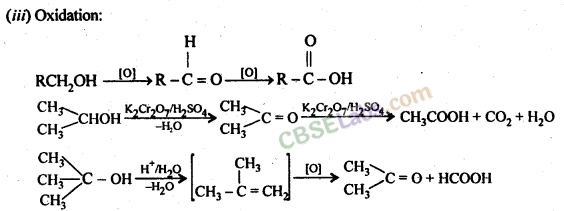
Chemical reactions of Alcohols and Phenols
(a) Reactions in which the O-H bond is cleaved
(b) Reactions involving the entire amount of alcohol.
Phenol reactions
Electrophilic substitution reaction
- The presence of the -OH group in phenols activates the aromatic ring towards electrophilic substitution and, due to the resonance effect, directs the incoming group to the ortho and para positions.
Kolbe’s reaction
- Sodium phenoxide is treated with C02 at 400K under 3-7 atm pressure to produce sodium salicylate, which is then acidified to produce salicylic acid.
Reimer-Tiemann reaction
- Salicyldehyde is produced when phenol reacts with chloroform in the presence of NaOH.
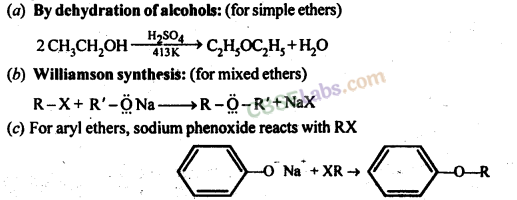
Ether Preparation
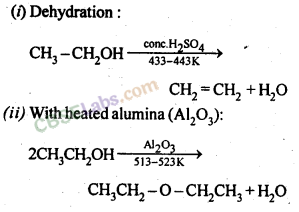
Physical and Chemical Properties
- Since ethers do not form intermolecular H-bonding, their boiling point is much lower than that of corresponding alcohols.
- Water soluble to a degree.
- The alkoxy group activates the aromatic ring and directs the incoming group to ortho and para positions in this electrophilic substitution.