CBSE Class 12 Chemistry Chapter 12 Revision Notes
Chapter 12: Aldehydes, Ketones and Carboxylic Acids Revision Notes
- Aldehydes, ketones, carboxylic acids, and their derivatives are organic compounds with the carbonyl group (CO) as the functional group.
- These are referred to as carbonyl compounds collectively.
- The carbonyl group’s oxygen atom is far more electronegative than the carbon atom.
- As a result, the oxygen atom attracts the electron cloud of the π-bond towards itself, resulting in an unsymmetrical π-electron cloud of >c = 0.
- As a result, carbonyl carbon has a positive charge while carbonyl oxygen has a negative charge.
- Hence, the carbonyl group is polar.
Aldehydes and Ketones Preparation Methods
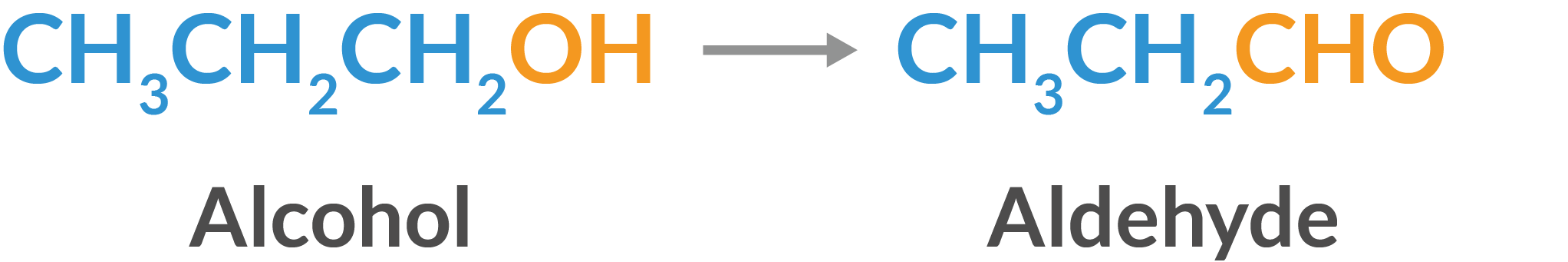
- Aldehydes and ketones are produced by the controlled oxidation of primary and secondary alcohol.
- Dehydrogenation of alcohols: Dehydrogenation of primary alcohols produces aldehydes, while dehydrogenation of secondary alcohols produces ketones.
Aldehyde Preparation
(a) Palladium on barium sulfate is used to hydrogenate acyl chloride (acid chloride), which is partially poisoned by the addition of S or quinoline. Rosenmund reduction is the name for this reaction. Aldehydes are made using this method.
(b) In the presence of hydrochloric acid, nitriles are reduced to corresponding imines, which are then hydrolyzed to give the corresponding aldehyde. Stephen’s reduction is the name for this reaction.
(c) Chromyl chloride (CrO2ClO2) oxidizes the methyl group of toluene to a chromium complex, which, when hydrolyzed, yields benzaldehyde. Etard reaction is the name given to this reaction.
(d) Benzaldehyde or substituted benzaldehyde is formed when benzene or its derivatives are treated with CO and HCl in the presence of anhydrous AlCl3 or CuCl. The Gatterman-Koch reaction is the name for this reaction.
Ketones Preparation
(a) Ketones are produced by treating acyl chlorides with dialkyl cadmium, which is made by reacting cadmium chloride with Grignard reagent.
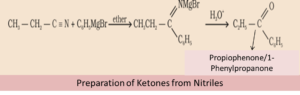
(b) From Nitriles
The corresponding ketone is formed when benzene or substituted benzene is treated with acid chloride in the presence of anhydrous A1C13. Friedel-acylation craft’s reaction is the name for this reaction.
Properties of Ketones and Aldehydes
- In nucleophilic addition reactions, aldehydes are much more reactive than ketones.
- Aldehydes and ketones undergo nucleophilic addition reactions with a variety of nucleophiles, including HCN, NaHSO3, alcohols, ammonia derivatives, and Grignard reagents, on the carbonyl group.
- Aldehydes and ketones are reduced to primary and secondary alcohols, respectively.
- Treatment with zinc amalgam and concentrated hydrochloric acid (Chemmenson reduction) or hydrazine followed by heating with NaOH or KOH in a high boiling solvent such as ethylene glycol reduces the carbonyl group of aldehydes and ketones to CH2 (Wolff-Kishner reduction).
- Aldehyde is oxidized by Tollen’s reagent (ammonical silver nitrate), and the silver ions are reduced to silver, which appears as a bright silver mirror on the test tube’s side. This test is not given by ketones.
- Aldehydes reduce Fehling’s solution, forming a red cuprous oxide precipitate. Fehling’s solution is made by combining a copper sulphate solution with a sodium hydroxide and sodium potassium tartrate solution. Fehling’s solution is not reduced by ketones. As a result, no precipitate forms.
- When warmed with dilute alkali, aldehydes and ketones with at least one α-hydrogen atom undergo a condensation reaction to form β-hydroxy aldehydes and β-hydroxy ketones, respectively. Aldol condensation is the name for this reaction.
- A mixture of four products results from the condensation of two different aldehydes or/and ketones, each with an a-hydrogen atom, in the presence of dilute alkali. Cross aldol condensation is the name for this reaction.
- Cannizzaro reaction: When aldehydes without an a-hydrogen atom are treated with concentrated alkali, they undergo self-oxidation and reduction (disproportionation). One molecule of aldehyde is reduced to alcohol white, while another is oxidized to carboxylic acid salt in this reaction.
- The carbonyl group acts as a deactivating and meta-directing group in the electrophilic substitution reaction, which takes place at the ring.
Methods of carboxylic acid preparation
(a) Primary alcohols and aldehydes are oxidized.
(b) Aromatic carboxylic acids can be made by oxidizing alkyl benzenes on the side chain.
(c) From nitrile and amide hydrolysis.
(d) Grignard reagents react with carbon dioxide to produce carboxylic acids.
- Due to the formation of hydrogen bonds with water, aliphatic carboxylic acids with up to four carbon atoms are miscible in water.
- As the number of carbon atoms increases, solubility decreases.
- The electron withdrawing group (Cl, NO2, CN, etc.) stabilizes the carboxylate anion by dispersing the carboxylate anion’s negative charge, RCOO–, increasing the acidic strength.
- The presence of an electron-donating substituent, such as an alkyl group, increases the negative charge on the RCOO– anion and destabilizes it, reducing the acidity of the carboxylic acid.
- In the presence of a small amount of red phosphorus, carboxylic acids with a α-hydrogen are halogenated at the α-position by treatment with chlorine or bromine, resulting in α- chloro or α– bromo carboxylic acids. The Hell-Volhard Zelinsky Reaction is the name given to this reaction.