CBSE Class 12 Chemistry Chapter 3 Revision Notes
Chapter 3: Electrochemistry Revision Notes
- Oxidation is defined as an electron loss, whereas reduction is defined as an electron gain.
- Both oxidation and reduction reactions occur simultaneously in a redox reaction.
- Both oxidation and reduction reactions take place in the same vessel in a direct redox reaction.
- In a direct redox reaction, chemical energy is converted to heat energy.
- Indirect redox reaction: Oxidation and reduction take place in separate vessels.
- Chemical energy is converted into electrical energy in an indirect redox reaction.
Electrochemical Cells
- An electrochemical cell is a device that converts chemical energy into electrical energy.
- The oxidation half-cell is an electrochemical half cell in which oxidation takes place.
- The reduction half-cell is the half-cell in which reduction occurs.
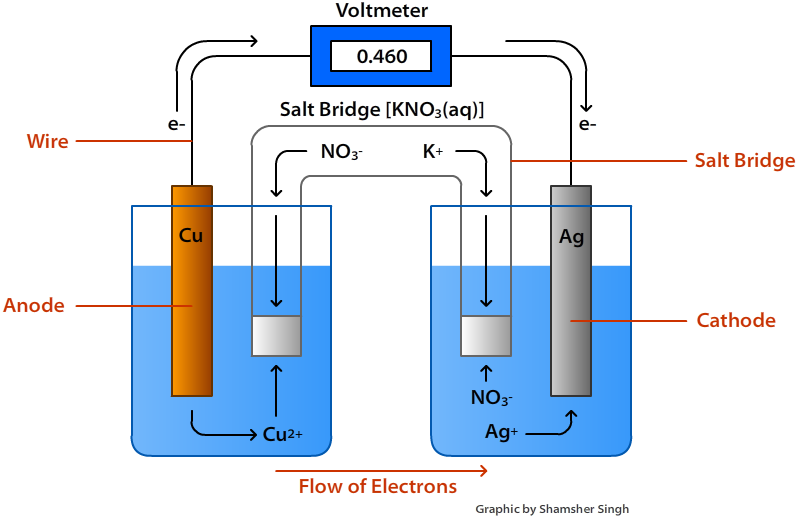
- The anode, which is negatively charged, undergoes oxidation, while the cathode, which is positively charged, undergoes reduction.
- Electric current flows in the opposite direction as electrons are transferred from anode to cathode.
- A metal plate is dipped into an electrolytic solution of its soluble salt to create an electrode.
- A salt bridge is a U-shaped tube made of agar-agar and gelatine that contains an inert electrolyte.
- By completing the electrical circuit, a salt bridge maintains electrical neutrality and allows the flow of electric current.
Electrode Potential
-
The potential difference that develops between the electrode and its electrolyte is known as electrode potential.
-
The potential difference between the metal and the solution of its ions is the result of charge separation at equilibrium. It’s a metric for how likely an electrode in a half cell is to lose or gain electrons.
-
Potential of a standard electrode: The electrode potential is known as standard electrode potential when the concentration of all species involved in a half cell is unity. E is the symbol for it.
-
Electrode potentials come in a variety of shapes and sizes. There are two kinds of electrode potentials: Oxidation potential and reduction potential
-
The tendency of an electrode to lose electrons or become oxidized is known as oxidation potential.
-
The tendency of an electrode to gain electrons or get reduced is known as reduction potential.
-
The inverse of reduction potential is oxidation potential.
-
The electrode with a higher reduction potential has a greater tendency to gain electrons, and thus functions as a cathode, whereas the electrode with a lower reduction potential functions as an anode.
-
An electrode’s standard electrode potential cannot be measured in isolation.
-
The Standard Hydrogen Electrode is used as a reference electrode and has a zero potential at all temperatures, according to convention.
-
SHE: A standard hydrogen electrode is made up of a platinum wire that is sealed inside a glass tube and has a platinum foil on one end.
-
The electrode is immersed in a beaker filled with an aqueous solution of an acid containing 1 mol of hydrogen ions.
-
At 298 K, hydrogen gas is continuously bubbled through the solution at 1 bar pressure.
-
The Platinum foil is where the oxidation or reduction takes place.
-
Both anode and cathode functions are possible with a standard hydrogen electrode.
-
A substance with a higher reduction potential value has a higher likelihood of being reduced. As a result, it’s a good oxidizer.
-
A substance with a lower reduction potential value has a higher likelihood of being oxidized. As a result, it works well as a reducing agent.
-
The cathode is the electrode with the highest reduction potential, while the anode is the electrode with the lowest reduction potential.
-
Cell potential is the difference in potential between the two electrodes of a galvanic cell, and it is measured in Volts.
-
The cell potential is the difference between the cathode and anode reduction potentials.
E cathode – E anode = E cell
- When no current is drawn through the cell, its potential is referred to as the electromotive force of the cell (EMF).
Nernst Equation
-
Nernst looked at how an electrode’s electrode potential changed with temperature and electrolyte concentration.
-
Nernst devised a formula for calculating the standard electrode potential EΘ and the electrode potential E.
-
Electrode potential rises as the concentration of the electrolyte rises and the temperature falls.
-
When applied to a cell, the Nernst equation aids in calculating the cell potential.
-
Ecteell’s cell potential is zero at equilibrium.
-
The equilibrium constant Kc and the standard cell potential EΘcell have the following relationship:
-
The decrease in Gibbs energy equals the work done by an electrochemical cell.
Conductance of Electrolytic Solution
-
Conductors are materials that allow electricity to pass through them.
-
Every conducting material obstructs the flow of electricity in some way, which is referred to as resistance. It is measured in ohms and is denoted by the letter R.
-
Any object’s resistance is proportional to its length l, and inversely proportional to its cross section area A.
-
Where the term ρ is used to denote specific resistance or resistivity.
-
Ohm meter is the SI unit for specific resistivity.
-
Conductance, G, is the inverse of resistance.
-
The ohm-1 or mho unit is used for conductance. It is also denoted by the letter S in Siemens.
-
Conductivity is known as the inverse of resistivity. It is symbolized by the symbol
.
- Sm-1 is the SI unit for conductivity.
Cell constant = Conductivity = Conductance
-
There are two issues with measuring the resistance of an ionic solution:
-
First, passing direct current alters the solution’s composition.
-
Second, unlike a metallic wire or a solid conductor, a solution cannot be connected to the bridge.
-
The problem of measuring the resistance of an ionic solution can be solved by using an alternating current source, and the second problem can be solved by using a conductivity cell, which is a specially designed vessel.
-
A conductivity cell is made up of two Pt electrodes that have been coated with Pt black.
-
They are separated by a distance ‘l’ and have an area of cross section A.
-
The molar conductivity of a solution is defined as: It’s the total conducting power of all the ions produced when 1 mole of an electrolyte is dissolved in solution.
-
Molar conductivity is the ability of a molecule to conduct electricity.
-
Conductivity equivalent: It is the conductivity of all the ions produced when one gram of an electrolyte is dissolved in solution. S cm2 (g equiv) -1 is the equivalent conductivity unit.
-
Kohlrausch’s Law of independent migration of ions: According to this law, an electrolyte’s molar conductivity can be expressed as the sum of individual contributions from its individual ions at infinite dilution.
-
Dissociation degree: It’s the ratio of molar conductivity at a particular concentration ‘c’ to molar conductivity at infinite dilution.
-
The Faraday constant is defined as follows: It’s the same as the charge on one mol of electrons. It is approximately equal to 96500 C mol-1 or 96487 C mol-1.
-
Faraday’s First Law of Electrolysis: During electrolysis, the amount of substance deposited is proportional to the amount of electricity passed.
-
Faraday’s Second law of Electrolysis: When the same charge is applied to different electrolytes, the amount of substance deposited is proportional to their respective weights.
Product of Electrolysis
- The electrolysis products are influenced by a variety of factors.
- The nature of the electrolyte and electrodes to be electrolyzed.
- Inert electrodes, such as platinum or gold, do not participate in chemical reactions, meaning they do not lose or gain electrons.
- When electrodes are reactive, they participate in chemical reactions and produce different results than inert electrodes.
- The oxidizing and reducing species‘ electrode potentials.
- Some electrochemical processes, while feasible but slow at lower voltages, necessitate extra voltage, i.e. voltage above that at which these processes will occur.
- Electrolysis products differ in terms of molten state and electrolyte aqueous solution.
Batteries
- Primary cells are the cells that make up the body. A primary cell, such as a Daniel cell, a dry cell, or a mercury cell, is one in which the reaction in the cell produces electrical energy. It is not rechargeable.
- Secondary cells are those that are used to store electricity, such as a lead storage battery or a nickel-cadmium cell. They are rechargeable.
- Nickel cadmium cell is a type of secondary cell that lasts longer than a lead storage cell but is more expensive to produce.