CBSE Class 12 Chemistry Chapter 5 Revision Notes
Chapter 5: Surface Chemistry
- The branch of chemistry that studies the phenomenon occurring at the surface or the boundary between two phases is known as surface chemistry.
- Adsorption is the process of a solid (or a liquid) attracting and retaining the molecules of a substance on its surface, resulting in a higher concentration of the molecules on the surface.
- The adsorbate is the substance that is adsorbed, and the adsorbent is the substance that adsorbs.
- Desorption is the removal of an adsorbed substance from the surface it is adsorbed to.
- Absorption is not the same as adsorption. The substance is uniformly distributed throughout the body of a solid or a liquid during absorption.
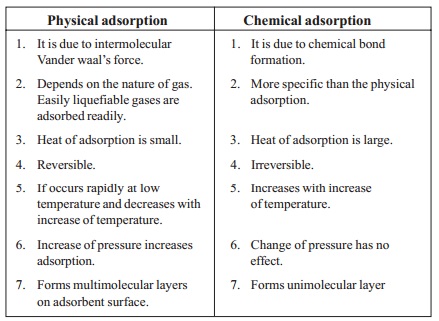
Types of Adsorption
- Physical adsorption or physisorption is a process in which the adsorbate is held on the surface by weak van der Waals forces. Heating or lowering the pressure can reverse this type of adsorption.
- Chemical adsorption or chemisorption occurs when the forces holding the adsorbate on the surface are comparable to chemical bond forces. This type of adsorption cannot be reversed.
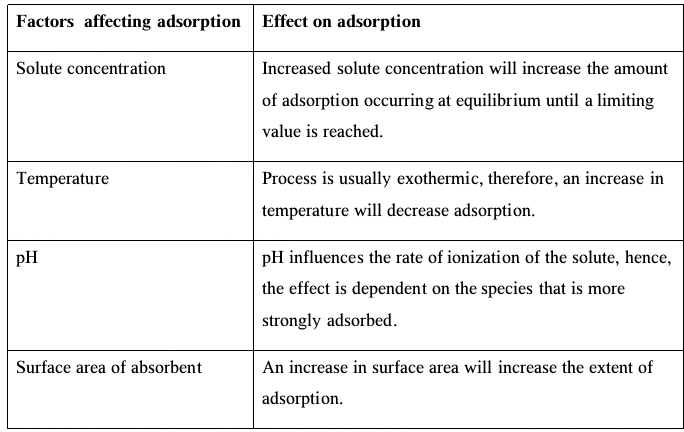
Factors that Affect Adsorption
- Adsorption is usually accompanied by the release of heat, making it an exothermic process.
- The extent of a gas’s adsorption on a solid is determined by the following factors:
-
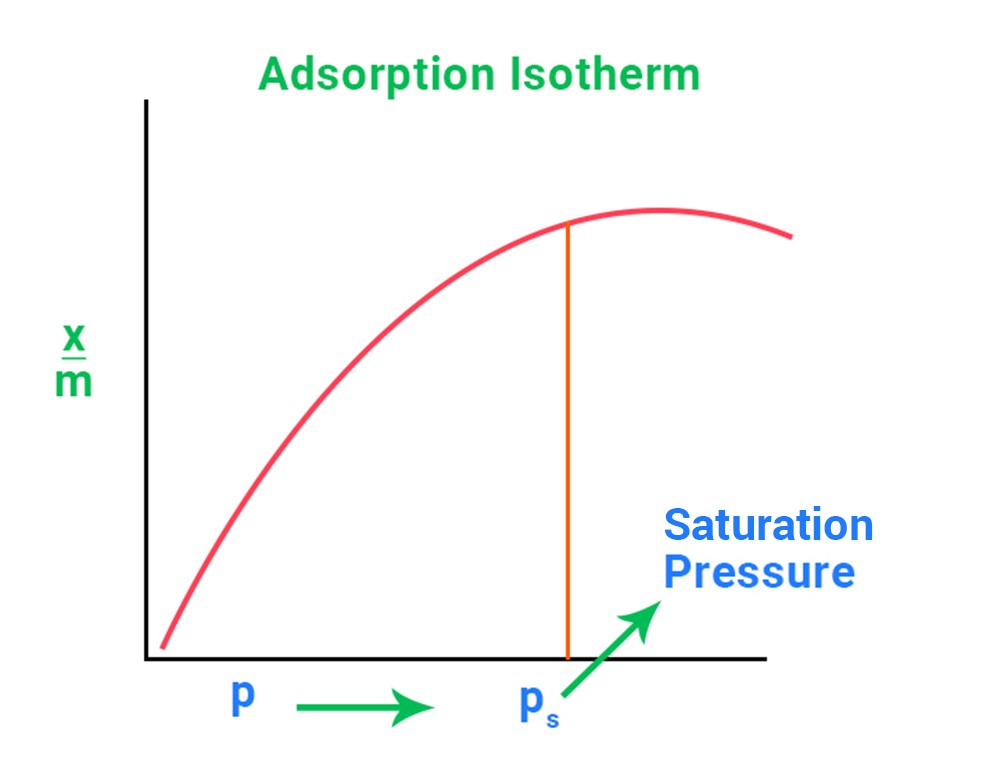
An adsorption isotherm is a relationship or graph between the magnitude of adsorption x/m and the pressure P of a gas at a constant temperature.
-
A straight line with a slope of 1 In will be plotted between log x/m and log P. It keeps well at room temperature. n = 1 at low pressure.
Applications of Adsorption
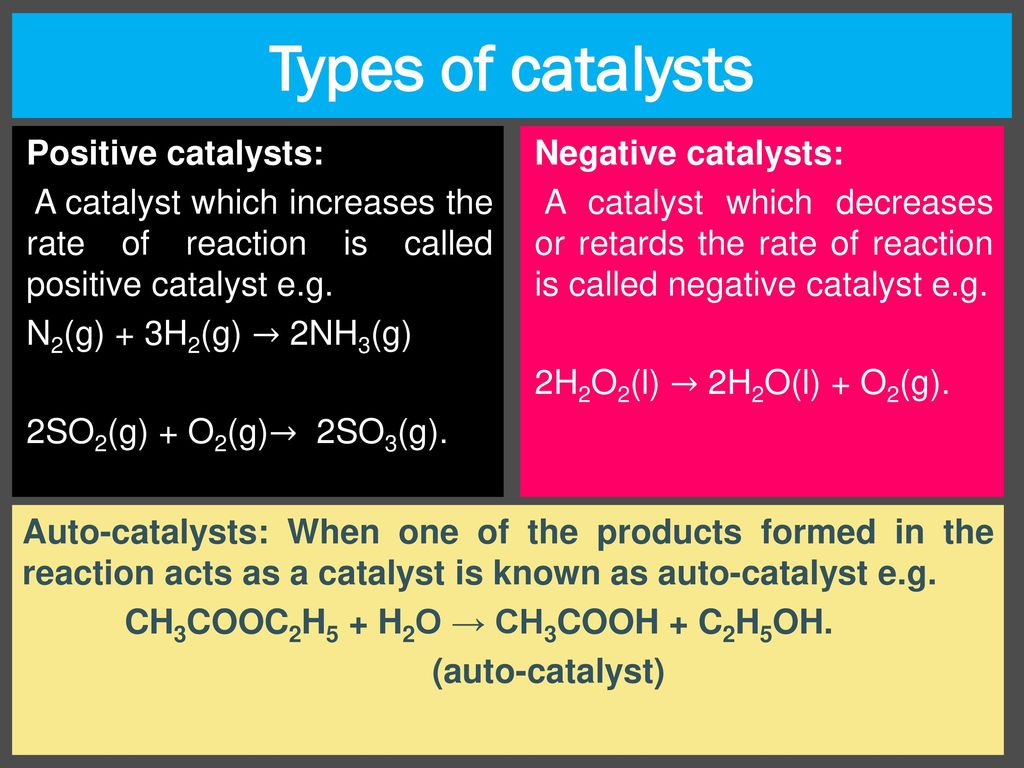
Catalysis
- A catalyst is a substance that can influence the rate of a chemical reaction while remaining chemically unchanged at the end.
- In homogeneous catalysis, the catalyst and the reactants are in the same phase.
- The catalyst is present in a different phase than the reactants in heterogeneous catalysis.
- Enzymes, also known as biological catalysts, are proteins that catalyze reactions in living organisms.
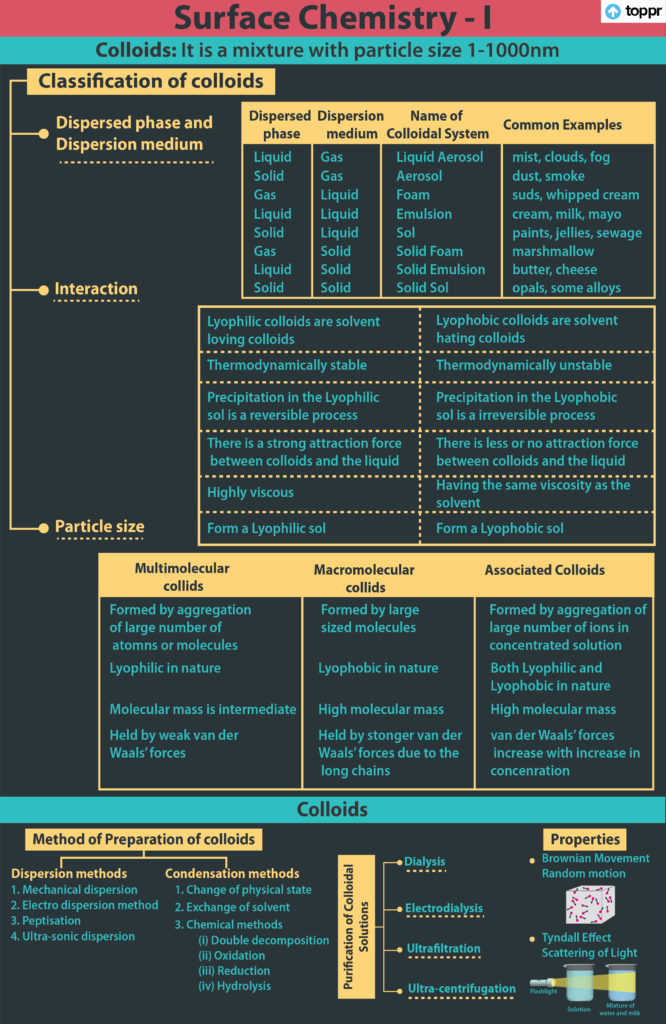
Colloidal Systems
- Colloidal solutions are a type of solution that falls somewhere between true solutions and suspensions. Colloidal particles range in size from 1 to 1000 nm in diameter.
- A colloidal system is a heterogeneous system made up of a dispersed phase and a dispersion medium.
- Colloidal systems are classified into eight categories based on the dispersed phase and dispersion medium.
Characteristics of Colloidal Solution
- Brownian movement refers to the zig-zag and random motion of colloidal particles.
- A light beam’s path becomes visible when it passes through a colloidal solution. The Tyndall effect is the name for this phenomenon. Colloidal particles scatter light, which causes this effect.
- Electrophoresis is the movement of colloidal particles in the presence of an electric field.
- Colloidal particles diffuse from a region of higher concentration to a region of lower concentration.
- Emulsions are colloidal systems in which the dispersed phase and the dispersion medium are both liquids, such as milk, which is made up of small drops of liquid fat dispersed in water.
- The process of creating an emulsion is called emulsification.