CBSE Class 12 Chemistry Chapter 6 Revision Notes
Chapter 6: General Principles and Processes of Isolation of Elements Revision Notes
- Minerals are the natural substances that occur in the earth and contain metals or their compounds.
- Ores are minerals from which metals can be extracted in a convenient and cost-effective manner.
- Native ores contain metals in their natural state, such as silver, gold, platinum, and other precious metals.
- Metallurgy refers to the entire process of extracting a pure metal from one of its ore.
- Ores typically contain soil, sand, stones, and other silicates that aren’t useful. The unwanted impurities found in ores are referred to as gangue or matrix.
- Ore-dressing, also known as ore concentration, is the process of removing unwanted earthy and siliceous impurities from ore, and the beneficiation process is the process of concentrating an ore.
- Ore concentration is achieved using (a) physical and (b) chemical methods.
Physical Methods
- Hand-picking: This method is used when the impurities are distinct enough from the ore to be distinguished with the naked eye.
- Hydraulic washing, Levigation, or Gravity Separation: The separation is based on the specific gravities of the gangue and ore particles being different.
- Electromagnetic separation: This method can be used to separate components that are magnetic in nature, such as ore or impurities.
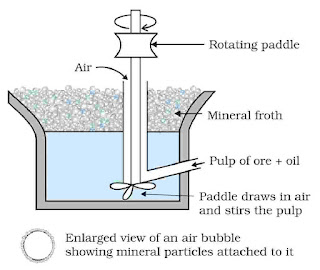
- Froth floatation process: For concentration of sulfide ores, this method is used.
Chemical Methods
- The chemical method (leaching) entails treating the ore with a suitable reagent to make it soluble while leaving impurities insoluble.
- A suitable chemical method is used to extract the ore from the solution.
Extraction
- Extraction is the process of extracting metals in their free state from concentrated ores.
- Chemical processes are used in the extraction of crude metal from concentrated ore.
(a) Ore conversion to metallic oxides
- In the absence of air, calcination involves heating the ore below its fusion temperature.
- It has the ability to remove moisture from hydrated oxide and CO2 from carbonates. It causes the ore to become porous.
- Roasting is the process of heating ore in the presence of air to temperatures below fusion!
(b) Free metal reduction
- Smelting is the process of reducing ore to molten metal at a high temperature. Powerful reducing agents such as C, H2 CO, Al, Mg, and others are used to extract electropositive metals such as Pb, Fe, and Sn.
- Auto-reduction process: This process is also known as self-reduction.
- Electrolytic process: Highly electropositive metal oxides, such as Na, K, Mg, Ca, Al, and others, are extracted by electrolysis of their oxides, hydroxides, or chlorides in a fused state. Electrolysis of alumina mixed with cryolite, for example, yields Al.
- Refining is the process of purifying the metals that have been extracted.
- The principle of chromatography is that different components of a mixture are adsorbed to different degrees on an adsorbent.