CBSE Class 12 Chemistry Chapter 7 Revision Notes
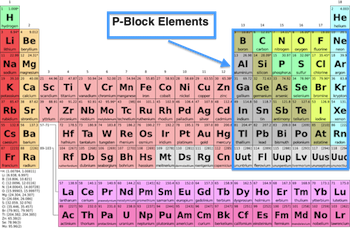
Chapter 7: The p-Block Elements Revision Notes
Group 15 Elements
- Nitrogen (7N), phosphorus (15P), arsenic (33As), antimony (51Sb), and bismuth (83Bi) are all found in group 15 of the Periodic Table.
- This group’s elements can have various oxidation states ranging from -3 to + 5.
- Nitrogen has a maximum covalency of four because it lacks d- orbitals to expand its covalency.
- The atomic (covalent) and ionic radii (in a given oxidation state) of nitrogen family (group 15) elements are smaller than those of carbon family elements (group 14).
- From N to P, the covalent radius increases dramatically. However, there is only a small increase from As to Bi.
- Nitrogen has a strong proclivity for forming pπ-pπ multiple bonds with itself, carbon, and oxygen.
- As we progress through the group, the likelihood of pπ-pπ multiple bonding decreases.
- Elements in group 15 are more electronegative than those in group 14. As you move down the group from N to Bi, the electronegativity decreases.
- Gaseous hydrides of the type MH3 are formed by all elements in group 15.
- As we progress through the group, the hydrides’ basic strength decreases. As a result, NH3 is the most powerful base. NH3 > PH3 > ASH3 > SbH3
- As the atomic size of the hydrides increases, their thermal stability decreases.
- Due to non-availability of d-orbitals, N cannot form NX5. Bi cannot form a BiX5 due to the unwillingness of its 6s electrons to participate in bond formation.
- Nitrogen breaks down into a variety of oxides. The remaining members of the group (P, As, Sb, and Bi) form two types of oxides: E203 and E205.
Group 16 Elements
- The elements oxygen (8O), sulphur (16S), selenium (34Se), tellurium (52Te), and polonium (84Po) are found in group 16 of the Periodic Table.
- The valence shells of the elements have the electronic configuration ns2np4.
- Due to its small size and high electronegativity, the first element of group 16 has a different chemical behavior than the other members of the group.
- As the atomic number rises, the metallic character rises with it. The first four elements have a nonmetallic quality to them. Non-metallic characters are strongest in O and S, weakest in Se and Te, and metallic in Po.
- As the number of shells increases,** the atomic and ionic radii increase** from top to bottom.
- As the group grows larger, the ionization enthalpy decreases.
- Elements in group 16 have lower ionization enthalpy values than elements in group 15. This is due to the extra stable half-filled p-orbital electronic configurations of group 15 elements.
- Due to the compact nature of the oxygen atom, it has a lower negative electron gain enthalpy than sulfur.
- O has the highest electronegativity value among the elements, second only to F. With increasing atomic number, electronegativity decreases within the group.
- As we progress through the group, the likelihood of catenation decreases.
- All the group’s elements form volatile hydrides.
- From water to hydrogen sulfide, the volatility rises and then falls.
- Their boiling point reflects this. H2S < H2Se < H2Te < H20 are the hydrides with the highest order of boiling points.
- Due to the increase in molecular weight, the group boiling point increases, which increases the van der Waal’s forces of interaction.
- Because of hydrogen bonding, H20 has an abnormally high boiling point.
- Hydride thermal stability decreases in the following order:H20>H2S>H2Se>H2Te>H2Po.
- The strength of the hydrides as acids increases in the following order: H20 < H2S< H2Se < H2Te
- Binary halides are formed by all of the elements in group 16.
- S, Se, and Te combine to form a variety of oxo-acids. Sulphuric acid is the most important of S’s oxo-acids.
- Sulphuric acid (H2S03) and thiosulfuric acid (H2S203) are unstable and therefore cannot be isolated. They can only be found in aqueous solutions or as their salts.
Group 17 Elements
- Fluorine (9F), chlorine (17Cl), bromine (35Br), iodine (53I), and astatine (55At) are all found in group 17 of the Periodic Table.
- For valence shells, the electronic configuration is ns2 np5.
- Due to maximum effective nuclear change, halogens have the smallest atomic radii in their respective periods.
- The initial ionization energies are relatively high, but they gradually decrease as the group progresses.
- Iodine can lose an electron, resulting in the formation of the I+ ion.
- Electron affinity varies as Cl > F > Br >I.
- F is the most electronegative element that has ever been discovered.
- Electronegativity decreases as you progress through the group.
- Halogens are excellent oxidizers. As you move down the oxidizing power of the group decreases.
- The order of reactivity is F2 > Cl2 > Br2 > I2.
- Hypohalous acids are all weak acids that can only be found in solution. The acid strength decreases as you progress through the group.
- For a given halogen atom, HOCl > HOBr > HOI
- Acid strength increases as the number of O-atoms increases. HOCl < HClO2 < HClO3 < HClO4
Group 18 Elements
- Helium (2He), neon (10Ne), argon (18Ar), krypton (36Kr), xenon (54Xe), and radon (86Rn) are all found in group 18 of the Periodic table.
- They are referred to as noble gasses as a group.
- The orbitals of their valence shell are completely filled.
- They are monoatomic and water soluble only in small amounts.
- The fluorides XeF2, XeF4, and XeF6 are formed by Xe.
- XeO3 has a square pyramidal shape, whereas XeOF4 has a trigonal pyramidal shape.
- Because he is a non-flammable gas that is lighter than air, He is used to fill meteorological balloons.
- Ar is used to create an inert environment.