Osmotic Pressure Study Guide
What is Osmotic Pressure
Almost every poet can’t help but praise the beauty of grown flowers in their poetry. A tulip garden is definitely a peaceful sight to see. As we all know, plants are living beings, and we can usually see them standing on their own, as they are an important part of our environment. But have you ever wondered how these plants manage to stay upright? Plants do not have bones to sustain their structure, like humans do. Osmotic pressure, a colligative property of solution, can answer this question.
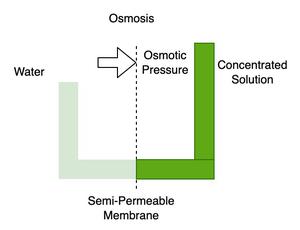
OSMOSIS
Colligative properties of solutions are determined by the concentration of solute molecules or ions and not by the identification of the solute. Vapor pressure reduction, boiling point elevation, freezing point depression, and osmotic pressure reduction are all examples of colligative qualities. These are the properties of solutions that are not dependent on the identity of the solute but are dependent on the concentration of the solute molecules.
The overall flow or passage of solvent molecules over a semipermeable membrane (one that allows the diffusion of particular molecules or ions through it ) through which solute molecules cannot pass is called osmosis. A net movement of solvent into the solution side of the membrane occurs when a sample containing both solute and solvent molecules is introduced on one side of the membrane, and the pure solvent is poured on the other.
Note: Solute particles cannot flow through the semipermeable barrier since it only permits solvent molecules to flow across.
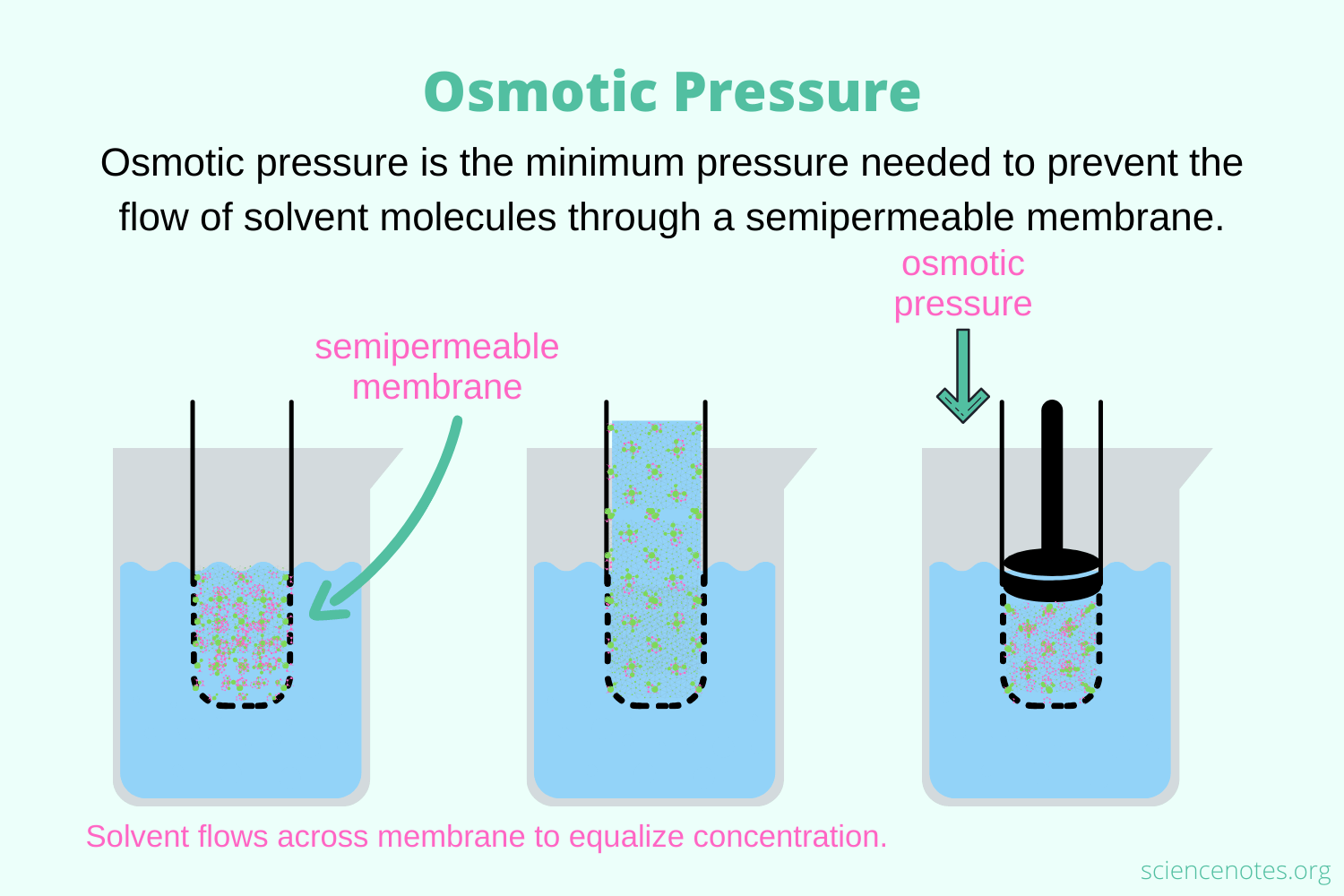
OSMOTIC PRESSURE
The osmotic pressure is the minimum pressure required to prevent the inward movement of a solution’s pure solvent across a semipermeable barrier. It can also be described as a measurement of a solution’s tendency to absorb a pure solvent via osmosis.
π = MRTwhere,
pi (π) is the osmotic pressure
M is the molar concentration
R is the gas constant
T is the temperature
Effects of Dehydration
Loss of water in cells and tissues of body is called dehydration. Dehydration can be caused when sufficient water is not available and there is high loss of fluid from the body. Dehydration can be caused in three stages – Mild Dehydration, Moderate Dehydration and Severe Dehydration.
Mild Dehydration
- After losing 1-2% of body fluid you will feel thirsty.
- Concentrated urine
- Shrunken cells in the brain might cause headache
- You will feel weak and exhausted
- Moderate Dehydration –
- It is pretty serious thing
- The cell shrinkage in the brain intensifies the pain and will make you feel tired and confused
- Dry mouth as saliva production is decreased
- Muscles will start to cramp
- Little to no urine
Severe Dehydration
- This can be life threatening
- Blood pressure drops extremely
- Blood flow to the vital organs is impaired
- Leads to organ failure, coma or death
- Temperature increase a person can get a shock and lose consciousness
Effects of over Hydration
Increased extracellular fluid volume due to pure water excess or water intoxication. It is often an induced condition.
Clinical Symptoms
Disordered Cerebral function, nausea, vomiting, headache, confusion and in severe cases coma and death.
Etiology
Excessive unmonitored intravascular infusion:
- Normal Saline (0.9% Sodium chloride)
- Ringers lactate
Renal Retention of Sodium and Water
- Congestive heart failure
- Acute glomerulonephritis
- Cirrhosis
- Cushing’s syndrome
- Chronic renal failure
Morphological Features
- Sudden weight gain
- Hematological and biochemical changes include reduced plasma electrolytes, lowered plasma proteins and reduced Packed Cell Volume (PCV)
APPLICATIONS OF OSMOTIC PRESSURE
Filtration (“reverse osmosis”), a typical water treatment method, is based on osmotic pressure. This is an extensively used water purification technology and is based on osmotic pressure. The filtered water is deposited in a chamber and subjected to a force greater than the osmotic pressure exerted by the water and any dissolved solutes.
CONCLUSION
-
The passage of solvent molecules through a semipermeable membrane from a low to a high solute concentration region is referred to as osmosis.
-
The osmosis process can be stopped if enough stress is put to the solution side of the semipermeable membrane. This stress is called osmotic pressure.
-
The following formula can be used to estimate osmotic pressure:
π = MRT
FAQs:
1. How do electrolytes affect colligative properties?
Electrolytes dissociate to add more solutes to the solution, changing the colligative characteristics significantly.
2. What are the colligative properties that explain why electrolytes have abnormally high values of colligative properties?
The properties of solutions that are not dependent on the identity of the solute but are dependent on the concentration of the solute molecules are termed colligative properties. Because the solute breaks down into ions in an electrolyte solution, the quantity of particles is higher, and the impact on colligative qualities will be higher as the number of ions increases.
We hope you enjoyed studying this lesson and learned something cool about Osmotic Pressure! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
]]>