CBSE Class 9 Science Chapter 1 Revision Notes
Chapter 1:Matter In Our Surroundings Revision Notes
- Matter is anything that takes up space, has mass, and can be sensed by the senses.
- Air, earth, fire, sky, and water are the five basic elements (the Panchtatva) according to an Indian ancient philosopher.
Characteristics of Particles of Matter
- Made up of minuscule particles.
- Between particles, there are empty gaps.
- Particles are always moving.
- Forces of attraction bind particles together.
Matter in its various states
Basis of Classification of Types
- Depending on the particle arrangement
- On the basis of particle energy
- Depending on the particle distance
There are five different states of matter.
- Solid
- Liquid
- Gas
- Plasma
- Condensate of Bose-Einstein
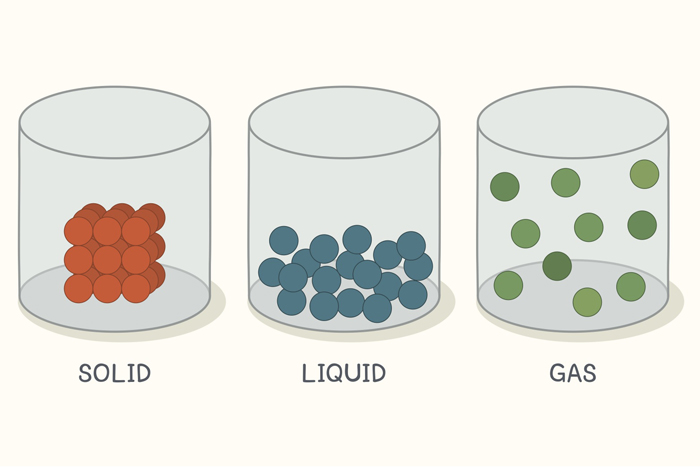
(I) SOLID
- The mass, volume, and shape of the object are all fixed.
- The shortest inter-particle distances exist.
- Incompressible.
- Do not diffuse and have a high density
- The strongest attraction forces exist between particles.
- The constituent particles are tightly packed.
(II) LIQUID
- There is no defined shape, but there is a fixed volume and mass.
- The spacing between particles are bigger than in solids.
- Almost impenetrable.
- The density of liquids is lower than that of solids, allowing them to diffuse.
- The attraction between particles is weaker than in solids.
- Particles in the constituents are less densely packed.
(III) GAS
- Neither the shape nor the volume are fixed.
- The distances between particles are the greatest.
- The material is extremely compressible.
- The density is the lowest and most diffuse.
- Attraction forces between particles are the weakest.
- Particles in the mixture are free to move about.
(IV) PLASMA
- An ionised gas is referred to as plasma.
- Magnetic fields impact plasma, which is a very good conductor of electricity.
- Plasma, like gases, has an amorphous shape and an amorphous volume. Ionized gas, for example.
(V) CONDENSATE BOSE-EINSTEIN
- BECs are states of matter that can exist at extremely low temperatures.
- In 1995, the scientists who worked on the Bose-Einstein condensate were awarded the Nobel Prize for their efforts.
- The BEC is concerned with molecules that are extremely near to one another (even closer than atoms in a solid).
Solid Property Explanation from a Microscopic Perspective
- Because the particles are fixed into place, solids have a defined shape and volume.
- Because the particles cannot move or slide past one another, solids do not flow smoothly.
- Because there is minimal empty space between particles, solids are difficult to crush.
Explanation of Liquid Properties at a Microscopic Level
- Because there is limited empty space between particles, liquids are difficult to compress and have a defined volume.
- Because the particles may move/slide past one another, liquids flow readily.
- Because the particles may move/slide past one another, liquids flow readily.
Microscopic Explanation of Gases’ Properties
- Because there is so much open space between particles, gases are easily compressed.
- Gases flow freely because particles pass through each other at random.
- Because particles can travel past one another (non-evaluative), gases have an unlimited shape and volume.
Explanation of Plasma Properties at the Microscopic Level
- Because the particles can move past one another, plasmas have an indeterminate shape and volume.
- Because there is so much open space between particles, plasmas are easily compressed.
- Because plasmas are made up of lenses, they are good conductors of electricity and are impacted by magnetic fields.
BEC Properties: A Microscopic Explanation
- Because particles exist at such low temperatures, they are less energetic than solids.
- Because they are trapped in the same space, particles are virtually indistinguishable.
- Because particles may move without friction in BEC, it demonstrates extreme fluidity.
Changes in the states of matter
- Water can exist in three states of matter: liquid, solid, and gas.
- As if made of ice, Water, for example, is a liquid, andAs water vapour, it is a gas.
- Sublimation is the transformation of a solid into vapours when heated and back to a solid when cooled. Ammonium chloride, camphor, and iodine are examples.
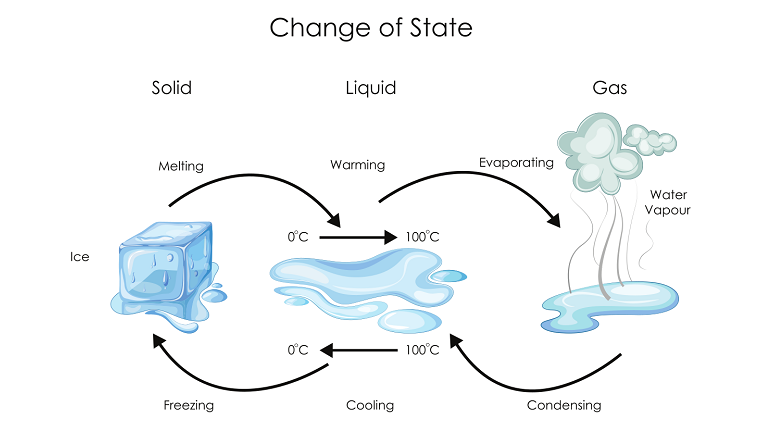
(a) The impact of temperature changes
- The influence of temperature on heating a solid varies based on the nature of the solid and the conditions under which the change must occur.
- As the temperature of solids rises, the kinetic energy of the particles rises, overpowering the forces of attraction between the particles, causing the solid to melt and become a liquid.
- The melting point of a solid is the temperature at which it melts to become a liquid at atmospheric pressure.
- Ice has a melting point of 273.16 K.
- Melting, or the transition from a solid to a liquid state, is also known as fusion.
(a) The Impact of Pressure Changes
- The condition of matter can be changed by increasing or lowering pressure. Gases can be liquefied by applying pressure and lowering the temperature.
- Under high pressure, solid carbon dioxide (CO2) is stored.
- When the pressure is reduced to 1 atmosphere, it is transformed to a gaseous state without passing through the liquid condition. This is why solid carbon dioxide is also referred to as dry ice.
- Heat that isn’t visible.
- During a transition of state, the concealed heat destroys the force of attraction between the molecules.
- Fusion To turn 1kg of solid into liquid, you’ll need a lot of heat.
- Vaporisation The amount of heat energy necessary to turn 1 kilogramme of liquid into a gas at atmospheric pressure at its boiling point
- As a result, we can say that pressure and temperature decide whether a thing is solid, liquid, or gas.
Boiling & Evaporation
- Matter particles are never at rest and are continuously moving.
- There are particles with variable quantities of kinetic energy in any gas, liquid, or solid at any given temperature.
- In liquids, a small fraction of particles at the surface with higher kinetic energy are able to break free from the forces of attraction of other particles and become vapour.
- Evaporation is the transformation of a liquid into vapours at any temperature below its boiling point.
Evaporation-Related Factors
- With an increase in surface area, the rate of evaporation increases.
- As the temperature rises, more particles gain enough kinetic energy to enter the vapour state.
- The amount of water vapour present in the air is referred to as humidity. At any given temperature, the air surrounding us can only carry a certain amount of water vapour. The rate of evaporation slows down when the amount of water in the air is already high.
- The higher the wind speed, the more evaporation occurs.
- Cooling is caused by evaporation.
- To make up for the energy lost during evaporation, liquid particles absorb energy from their surroundings.
Boiling vs. Evaporation
- Boiling is a phenomenon that affects a large number of people. Particles from the liquid’s bulk (whole) transition to a gaseous condition.
- Evaporation is a phenomena that occurs at the surface of the water. Particles from the surface accumulate enough energy to overcome the liquid’s attraction forces and transition to the vapour stage.
Kelvin and Celsius Temperature Scales
- Kelvin is the SI unit of temperature.
- 0 degree celsius = 273.16 K
- Because the Kelvin scale of temperature always has a positive sign, it is considered to be a superior scale than Celsius.
- The atmosphere (atm) is a measurement unit for the pressure exerted by a gas. Pascal (Pa) is the SI unit of pressure: 1 atmosphere = 1.01 (10 to the power 5) Pa.
- Atmospheric pressure is the pressure of air in the atmosphere. At sea level, the atmospheric pressure is 1 atmosphere, which is used to determine the normal atmospheric pressure.
