CBSE Class 9 Science Chapter 2 Revision Notes
Chapter 2: Is Matter Around Us Pure Revision Notes
MIXTURE vs. PURE SUBSTANCE
- A pure material is made up of only one type of substance.
- Two or more pure ingredients make up a mixture.
- Physical processes cannot differentiate pure substances from other substances.
- Physical procedures can be used to separate a mixture into its constituents.
- Each pure substance has distinct qualities.
- The qualities of the components of a mixture are displayed.
- Only one type of atom makes up an element. A compound is made up of only one type of molecule.
Different Mixture Types
i) Mixtures can also be classified according to the physical states of each component.
Sugar crystals in water are an example of a solid-liquid mixture. And dust in the air can be a mixture of solid and gas.
ii) on the grounds of incompatibility:
Homogenous Mixture
- It is made up of only one phase.
- Composition that is similar
- Sugar dissolved in water, for example.
Heterogeneous Mixture
- It is made up of two or more phases.
- Its makeup is not consistent.
- Air, sand, and common salt are three examples.
Separating a mixture’s components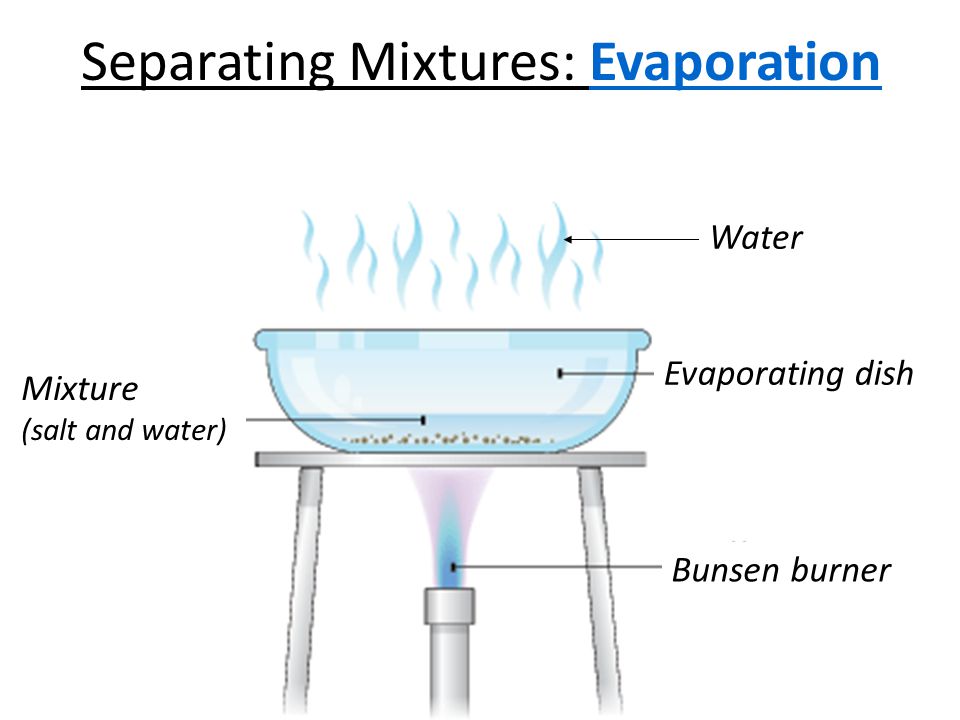
- Simple procedures such as hand picking, sieving, and winnowing can be used to separate the components of a heterogeneous mixture.
- Evaporation is a process that uses a mixture of salt and water or sugar and water.
- Butter from curd, fine mud particles suspended in water, centrifugation
- Oil is separated from water via decantation (using a separating funnel).
- Camphor from salt is sublimated.
- Different pigments extracted from flower petals were chromatographed.
- Distillation and fractional distillation are methods for separating constituents of a mixture. Petroleum
- Iron pins separated from sand by magnetic separation.
Different Types of Solutions
a) based on the size of the solute particles
True Solution
- Homogeneous
- The size of the solute particles is smaller than 1 nanometer (nm) or less.
- Filter paper is impenetrable to particles.
- Sodium chloride in water, sugar, and water in a stable solution
Sol[Colloid]
- Heterogeneous
- The size of solute particles ranges from 1 nm to 10 nm.
- Filter paper is impenetrable to particles.
- Only on centrifugation does it become stable and settle.
- Fog, Milk
Suspension
- Heterogeneous
- Particles with a diameter more than can’t pass through filter paper.
- Unstable at first, but eventually settles down.
- Water, chalk, and muddy water There is smoke in the air.
A heterogeneous mixture is a colloidal solution. It is divided into two parts:
(i) Dispersed phase: a component that is present in a minor amount.
(ii) Dispersion medium: a high fraction of a component is present.
Colloid particles are large enough to scatter and make visible a beam of light passing through it. As a result, they exhibit the Tyndall effect.
The colloidal particles are traveling in a zigzag pattern in all directions at random.
Brownian movement refers to the zig-zag motion of colloidal particles.
b) based on the amount of solute:
Unsaturated solution — An unsaturated solution is one that has a lower amount of solute that it can dissolve at a given temperature.
Saturated solution- A saturated solution is one that has the maximum quantity of solute it can dissolve at a given temperature.
Super saturated solution- Supersaturated solution is a solution that may dissolve a large amount of solute by raising the temperature of a saturated solution.
c) on the basis of the solvent’s nature
- Aqueous solution is a solution in which the solvent is water.
- A non-aqueous solution is one in which the solvent is not water (such as ether, alcohol, or acetone).
Changes in Physical and Chemical Properties
Physical changes
- are those that occur but do not result in the creation of a new substance.
- You still have at the conclusion of the change if you melt a block of ice.
- You still have glass if you smash a bottle.
- Melting, freezing, condensing, breaking, crushing, cutting, and bending are some examples.
Chemical transformations
- Transformations that result in the synthesis of a new material.
- A shift in hue, like that of autumn leaves, is a sign of a chemical alteration.
- a dark apple that has been half-eaten
Alloys
- A material with metallic qualities that is made up of two or more chemical components, at least one of which is a metal.
- Physical methods cannot separate these into their constituent parts.
- These, on the other hand, are termed mixtures since they display the features of their elements and can have a changeable composition.
- The advantage of alloys is that they allow you to combine metals with different properties to create a final product that is stronger, more flexible, or otherwise appealing to manufacturers.
- Aluminum alloys are widely employed in the manufacture of automotive engine components.
- Copper alloys have good electrical and thermal properties, as well as good corrosion resistance, ductility, and a low cost.
- Many commercial applications, such as watch straps and flatware, use stainless steel alloys.
- Titanium alloys are employed in aircraft structures because of their great strength, hardness, and stiffness.
Source:
]]>