CBSE Class 11 Biology Chapter 9 Revision Notes Part 2
Multiple Choice Questions
-
A disaccharide is formed when two monosaccharides are bonded together by a _________ bond.
-
Which of these nitrogen bases does RNA lack? _____________
-
Which of the following chemical classes does not belong to the vast group of carbohydrates? ____________
-
Glycosidic linkage is an ___________
Biomolecules Part 2
- Our human bodies are made up of trillions of cells that collectively perform various life functions.
- The various cells of the body can help in performing these functions with the help of organic molecules, which are ultimately known as biomolecules.
- Biomolecules are lifeless and complex organic molecules that combine in a specific manner and help in producing life and controlling biological reactions.
What are macromolecules?
- Macromolecules are very large molecules like proteins composed of hundreds of atoms made up of covalent bonds.
- Many of these molecules are polymers as they are made up of smaller molecules called monomers.
- Some of the most common macromolecules in biology are biopolymers and large nonpolymeric molecules like lipids and macromolecules.
- All macromolecules are homogeneous or heterogeneous molecules of many simple organic compounds.
Types of macro biomolecules
There are 4 major types of bio macromolecules, they are:
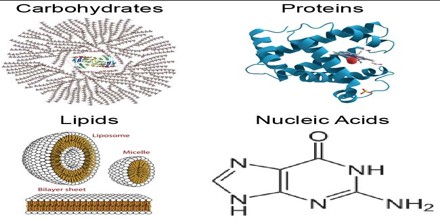
Source: Biomolecules of life
I) Proteins
- They perform important functions in living beings.
- They are present as hormones – insulin, enzymes – lipases, tissue fibers – elastin, collagen, etc.
- Many molecules of protein also take part in transportation through membranes like the GLUT 4, and they also help fight infections and antibodies.
- Proteins are heteropolymers made up of amino acids.
- Linear protein molecules have sequences of amino acids put together by peptide bonds.
- Thus, proteins are also called polypeptides.
Proteins are divided into 4 groups based on their structure:
-
Primary structure: The structure formed by peptide bonds within amino acids.
-
Secondary structure: These are folded structures between polypeptides by forming hydrogen bonds between the carboxyl oxygen groups. They include structures like the beta-sheets as well as an alpha helix.
-
Tertiary structure: They are 3-dimensional conformations formed because of interactions between the R groups of amino acids. The bonds that help in these structure formations are hydrophobic, the Van de Wall interaction forces, and hydrogen bonds.
-
The quaternary structure: is formed between more than 2 peptide chains, and they generally occur within identical and different polypeptide chains. The bonds that help in these structure formations are hydrophobic, hydrogen bonds, Van de Wall forces of interaction, and covalent crosslinks.
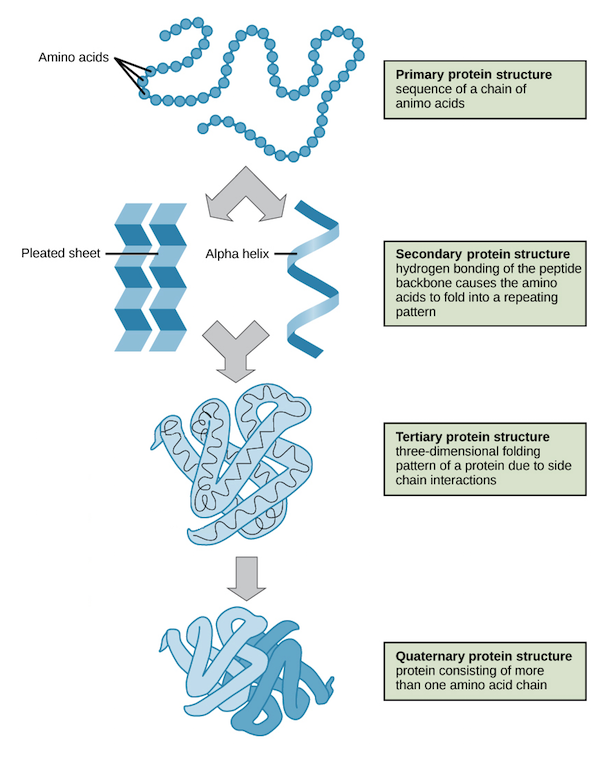
Source: protein structure
II) Nucleic Acids
- They are found in all living cells.
- Even viruses contain nucleic acids like DNA as well as RNA. They also contain genetic information.
DNA
- Watson and Crick gave this double-helical model.
- DNA consists of antiparallel strands of polynucleotide chains coiled around each other in the right direction.
- DNAs are formed by phosphate groups and sugars with nitrogenous bases.
- The nitrogenous bases of 1 strand are attached to those present in the other strands.
- Adenine pairs with guanine forming 2 hydrogen bonds, and cytosine with thymine forming 3 hydrogen bonds.
RNA
- They are single-stranded polynucleotide chains that contain ribose sugars and **uracil **in place of thymines.
- There are 3 types of RNA, tRNA, mRNA, and rRNA.
- mRNA provides templates for protein synthesis.
- Ribosomes are also RNA molecules. They act as enzymes and catalyze biochemical reactions.
III) Carbohydrates
- Carbohydrates are polymers of monosaccharides or simple sugars, and they are the main sources of energy in plants and animal cells.
- Carbohydrates are classified into 3 groups, they are:
- Monosaccharides
- They are made of single units of polyhydroxy aldehydes and ketones.
- These solids are colorless and crystalline soluble in water, and they help generate energy in the body.
- Examples are glucose, fructose, etc.
- Disaccharides
- These compounds consist of 2 units of sugars joined by O-glycosidic bonds.
- They consist of monomer units.
- Examples are: Sucrose, lactose, maltose, etc.
- Polysaccharides
- These compounds consist of more than 2 sugar monomer units known as glycans.
- They are of 2 types – Structural polysaccharides provide mechanical support to cells, organs, and organisms.
- Storage polysaccharides serve as carbohydrate stores releasing sugar monomers when required by the bodies. Examples are insulin, starch, etc.

Source: Difference between monosaccharides and polysaccharides
IV) Lipids
- Lipids play very important roles in metabolism.
- They are high-energy-yielding compounds that are water-soluble and found in membranes.
- They are also found as hormones and energy reserves.
- Fats, steroids, cholesterol, oil, steroids, etc., are all examples of lipids.
- They are soluble in various organic solvents such as benzene, ether, etc.
Various classes of lipids are as follows:
Fatty acids
- They are the most simple form of lipids.
- Being made of hydrocarbon chains and 1 acid group, they may be branched and linear.
- They are also known to be building blocks of other lipids.
Waxes
- They are esters of fatty acids and long-chained alcohols.
- They are made of 14-36 hydrocarbon chains.
- Plants, as well as animals, synthesize these waxes.
- A famous example is bee wax.
Steroids
- They are the complex derivatives of triterpenes.
Glycolipids
- They are lipids that consist of groups of saccharides.
- They are important constituents of cell membranes and also help in transmitting signals.
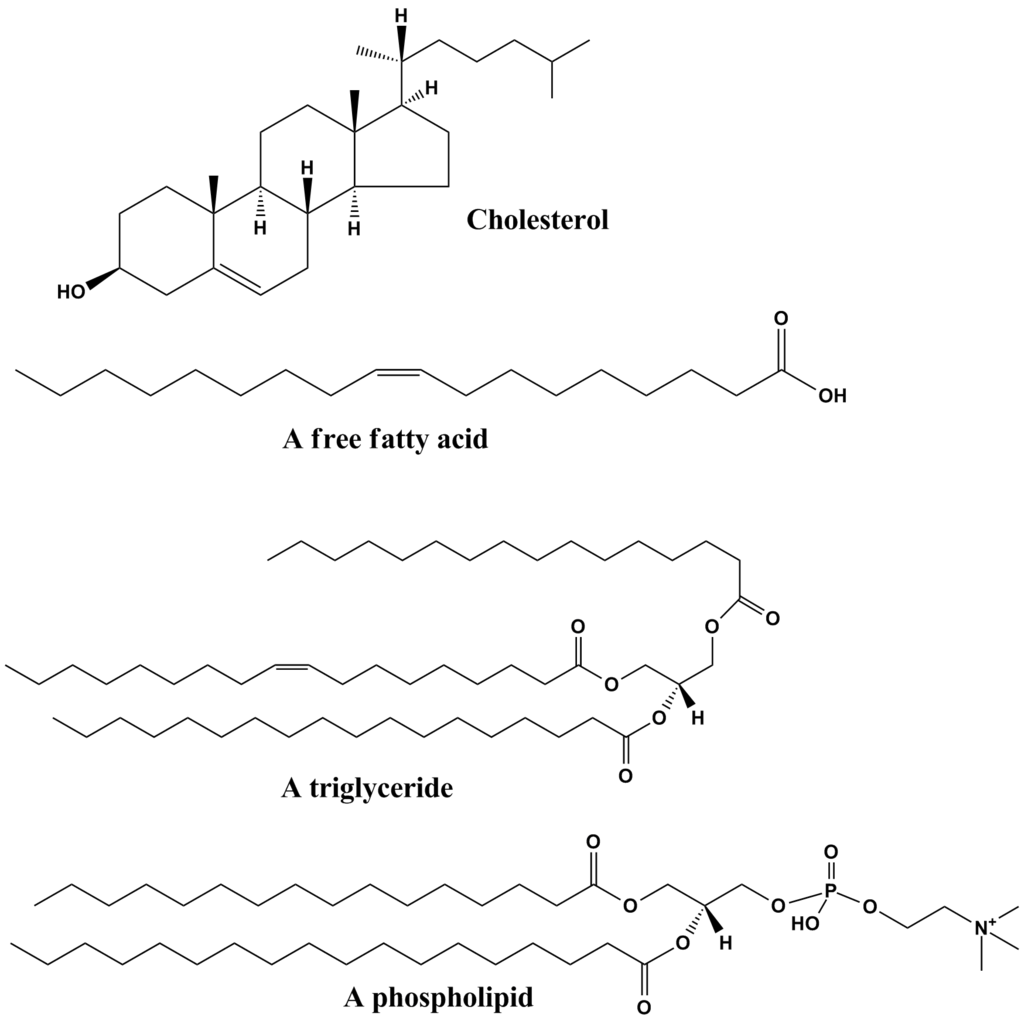
Source: Common lipid structures
Nature of bond linking polymer
- In polypeptides or proteins, amino acids are linked by peptide bonds formed when the carboxyl (-COOH) groups of one amino acid react with the amino (-NH2 ) groups of the next amino acid with the elimination of water – this process is called dehydration.
- In polysaccharides, the individual monosaccharides are linked by glycosidic bonds.
- The dehydration process also forms these bonds.
- These bonds are formed between two carbon atoms of two adjacent monosaccharides.
- In a nucleic acid, a phosphate links the 3’-carbon of one sugar of one nucleotide to the **5’-carbon **of the sugar of the succeeding nucleotide.
- The bond between the phosphate and hydroxyl group of sugar is an ester bond.
- As there is one such ester bond on either side, it is called the phosphodiester.
- Nucleic acids exhibit a wide variety of secondary structures.
- For example, one of the secondary structures exhibited by DNA is the famous Watson-Crick model.
- This model says that DNA exists as a double helix.
- The two strands of polynucleotides are antiparallel, running in the opposite direction.
- The backbone is formed by the sugar-phosphate-sugar chain.
- The nitrogen bases are projected perpendicularly to this backbone but face inside.
- A and G of one strand compulsorily base pairs with T and C, respectively, on the other strand.
Enzymes
- Most of the enzymes are proteins.
- Some nucleic acids behave like enzymes which are called ribozymes.
- An enzyme, like any protein, has a primary structure, i.e., the amino acid sequence of the protein.
- Enzymes also have a secondary and tertiary structure.
- The backbone of the protein chain folds upon itself, the chain crisscrosses itself and hence, many crevices or pockets are made.
- One such pocket is the ‘active site’.
- An active site of an enzyme is a crevice or pocket into which the substrate fits.
- Thus enzymes, through their active site, catalyze reactions at a high rate.
- Enzyme catalysts differ from inorganic catalysts in many ways, but one major difference is that inorganic catalysts work efficiently at high temperatures and pressures, while enzymes get damaged at high temperatures.
- However, enzymes isolated from organisms that normally live under extremely high temperatures (e.g., hot vents and sulfur springs), are stable and retain their catalytic power even at high temperatures (upto 80°-90°C).
Nature of Enzyme Action
-
Each enzyme (E) has a substrate (S) binding site in its molecule so that a highly reactive enzyme-substrate complex (ES) is produced.
-
This complex is short-lived and dissociates into its products P and the unchanged enzyme with an intermediate formation of the enzyme-product complex (EP).
-
The formation of the ES complex is essential for catalysis.
E + S ES → EP → E + P -
The catalytic cycle of enzyme action can be described in the following steps:
- The substrate binds to the active site of the enzyme, fitting into the active site.
- The binding of the substrate induces the enzyme to alter its shape, fitting more tightly around the substrate.
- The active site of the enzyme, now in close proximity to the substrate, breaks the chemical bonds of the substrate and the new enzyme-product complex is formed.
- The enzyme releases the products of the reaction, and the free enzyme is ready to bind to another molecule of the substrate and run through the catalytic cycle once again.
Factors Affecting Enzyme Activity:
- Temperature and pH
- Concentration of Substrate
Co-factors
- Enzymes are composed of one or several polypeptide chains.
- There are a number of cases in which non-protein constituents called cofactors are bound to the enzyme to make the enzyme catalytically active.
- In these instances, the protein portion of the enzymes is called the apoenzyme.
- Three kinds of cofactors may be identified: prosthetic groups, coenzymes, and metal ions.
- Prosthetic groups are organic compounds and are distinguished from other cofactors in that they are tightly bound to the apoenzyme.
- For example, in peroxidase and catalase, which catalyze the breakdown of hydrogen peroxide to water and oxygen, haem is the prosthetic group and it is a part of the active site of the enzyme.
- Coenzymes are also organic compounds, but their association with the apoenzyme is only transient, usually occurring during the course of catalysis.
- Furthermore, coenzymes serve as cofactors in a number of different enzymes catalyzed reactions.
- The essential chemical components of many coenzymes are vitamins, e.g., coenzyme nicotinamide adenine dinucleotide (NAD), and NADP contain the vitamin niacin.
- A number of enzymes require metal ions for their activity which form coordination bonds with side chains at the active site and at the same time form one or more coordination bonds with the substrate, e.g., zinc is a cofactor for the proteolytic enzyme carboxypeptidase.
- Catalytic activity is lost when the cofactor is removed from the enzyme which testifies that they play a crucial role in the catalytic activity of the enzyme.
