Diffusion and Osmosis Study Guide
Introduction
People are frequently required to describe the commonalities and dissimilarities between osmosis and diffusion and try and compare the two modes of transportation. To respond to the topic, one needs to understand the meaning of osmosis and diffusion.
Osmosis
To equalize concentration, solvent particles are moved through a semipermeable membrane from a dilute solution into a concentrated solution. The solvent flows across the membrane to dilute the concentrated solution and balance the concentrations on both sides of the membrane. The entire procedure does not involve the use of energy. This process is called osmosis.
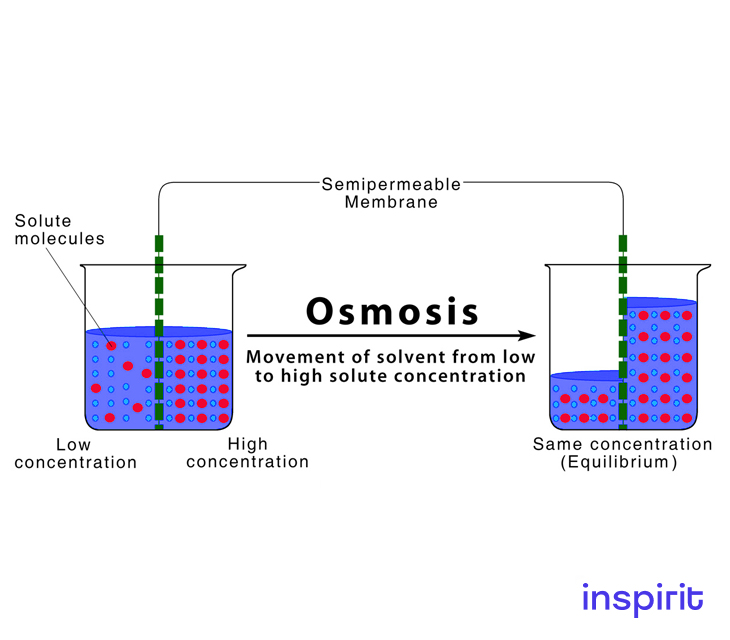
Osmosis Examples
-
The absorption of water molecules from the soil by the plant’s roots.
-
Red blood cells swell when exposed to water.
-
Soak gummy sweets in water to observe a simple example of osmosis. The gel in them functions as a semipermeable barrier.
The movement of water and osmosis
-
Water diffuses through cell membranes, a process known as osmosis.
-
Osmosis is the passage of water through a semipermeable membrane, with the solvent (for example, water) migrating from a low solute (dissolved substance) concentration area to a high solute concentration area.
-
In this scenario, the semipermeable barrier prevents the solute from passing through.
-
This is similar to diffusion because it includes the same random mechanism as water traveling along its concentration gradient.
-
Therefore, water will flow from the hypotonic solution to the hypertonic solution until the concentrations of the solutes are equal.
The plant cell and osmosis
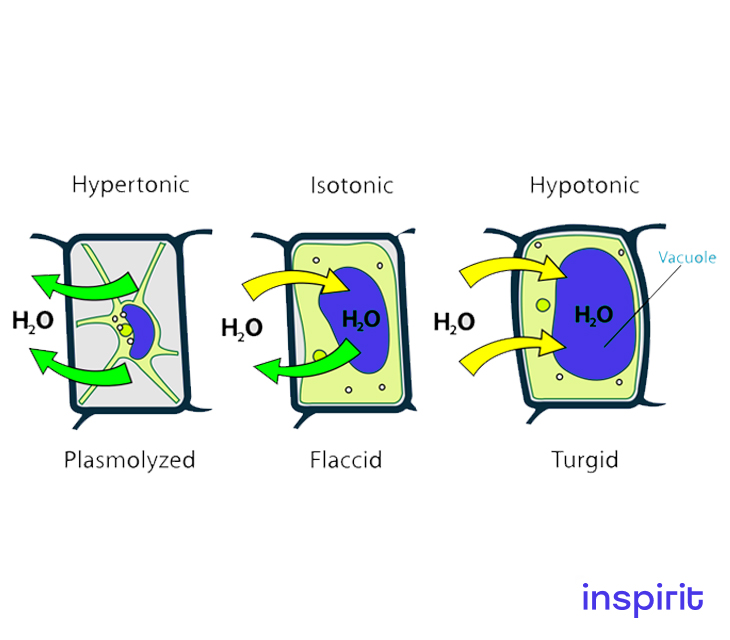
-
Osmosis is a process by which plants receive water from their roots and leaves, even if they are several feet above ground level.
-
Plants transfer sugars and other dissolved substances to their roots to create a gradient between the interior and exterior of the root; water from the ground then flows in by osmosis.
-
Water is then drawn up the tubes inside the plant called the xylem and evaporates out the leaves through to a process called transpiration.
-
Once created, this water column should remain intact for the duration of the plant’s life.
-
This naturally occurring phenomenon has aided in the development of useful technology. Water purification is one example.
NASA has just begun to investigate the use of advanced osmosis to purify and reuse wastes onboard the International Space Station, as well as for terrestrial uses. Semipermeable membranes are used to remove pollutants from water, making it drinkable. This technique was recently used to help rescue operations during a catastrophic flood in Western Kenya.
Diffusion
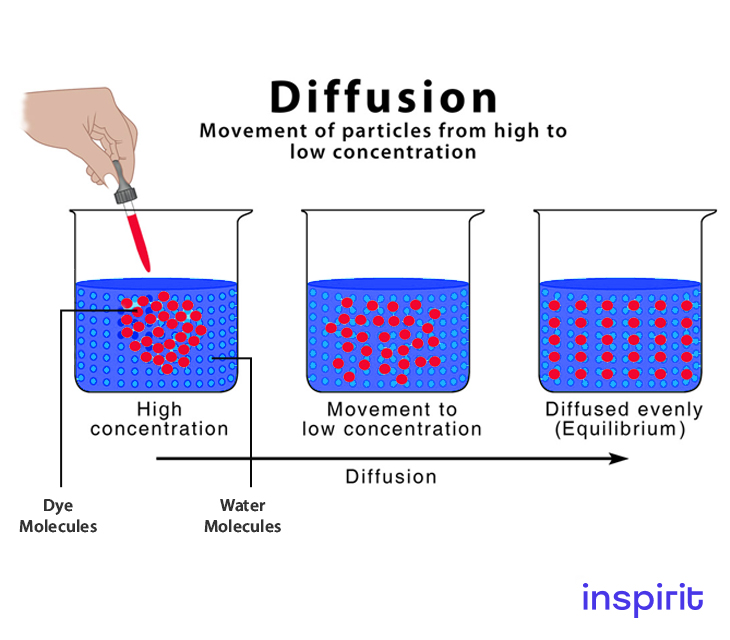
Diffusion is the process that involves the movement of particles from a high concentration area to a low concentration area. The movement of particles happens until equilibrium is attained.
Simple diffusion does not necessitate energy use; however, assisted diffusion necessitates the use of ATP. The principal energy transporter in cells is adenosine triphosphate or ATP.The ultimate effect during this process is to balance the concentration across the medium.
Facilitated Diffusion
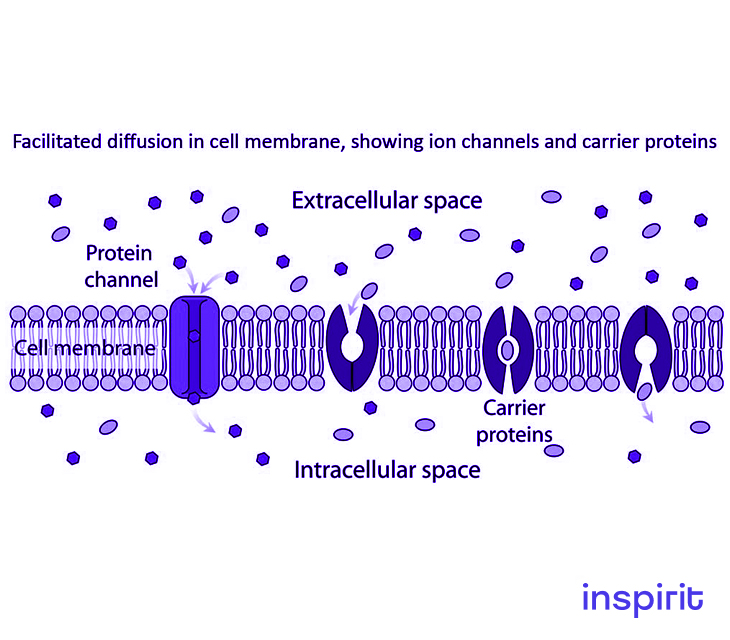
Facilitated diffusion is categorized under diffusion. It is the passive movement or transport of molecules or ions across a biological membrane.
This process is selective since the membrane allows the passage of only selective molecules and ions. It takes place via specific transmembrane integral proteins which are supported by electric charge and pH that aids in diffusion across the membrane.
Facilitated diffusion is one form of passive transport referring to a cellular transport mechanism where substances move along their concentration gradient.
Diffusion Examples
-
The scent is dispersed in the air when the perfume is sprayed.
-
The displacement of tiny molecules across cellular membranes.
-
Adding a drop of food coloring to water is one of the simplest demonstrations of diffusion. Though various transport mechanisms exist, diffusion is the most important.
Diffusion and cell membrane
-
Cells must transfer materials into and out of their cytoplasm via their cell membranes to operate.
-
These membranes are semipermeable, which means certain particles can flow through, but not others.
-
The phospholipid bilayer and its embedded proteins mediate this flow of molecules, some of which operate as transport channels for molecules that would otherwise be unable to pass across the membrane, such as electrons and carbohydrates.
Osmosis and diffusion: The differences
| Osmosis | Diffusion |
|---|---|
| It is only applicable to the liquid medium. | It can be found in liquids, gases, and even solids. |
| A semipermeable membrane is required. | It does not necessarily use a semipermeable |
| This is determined by the number of solute particles dissolved in the solvent. | It is affected by the presence of other particles. |
| Water is required for particle dispersion. | The movement of particles does not need water. |
| Only the solvent molecules can diffuse. | Solute and solvent molecules can both disperse. |
| Particles can only flow in one direction. | The movement of particles happens in all directions. |
| The entire process may stop or reverse by adding more pressure to the solution side. | This process cannot be interrupted or altered. |
| Only occurs between solutions of the same kind. | Occurs between solutions that may be similar or different. |
| It comprises only the transfer of solvent molecules from one side to the other. | It entails moving all of the particles from one place to another. |
| The solvent concentration does not become equal on both sides of the membrane. | The diffusion substance’s concentration equalizes to fill the available area. |
| Depends on solute potential. | It is not affected by solute potential, pressure potential, or water potential. |
| Only water or another solvent flows from a high-energy or density zone to a low-energy or density zone. | Any material can travel from a high energy or density location to a region of low energy or density. |
| It has nothing to do with mineral and vitamin absorption. | It aids in the absorption of minerals and nutrients. |
Osmosis and diffusion: Similarities
-
Osmosis and diffusion are processes that share certain characteristics.
-
The concentration of two liquids is equalized via both osmosis and diffusion.
-
Diffusion and osmosis are both passive transport processes, which means they do not require any additional energy input to proceed.
-
In both diffusion and osmosis, particles travel from a high-concentration location to a low-concentration location.
Conclusion
Diffusion and osmosis are membrane transport processes that equalize a solution’s content. Diffusion occurs when particles travel from a high-concentration area to a low-concentration area until equilibrium is attained. Because osmosis uses a semipermeable membrane, only the solvent molecules can migrate to equalize concentration.
-
There are two types of transport systems that integrate the flow of molecules into and out of the cell, namely osmosis and diffusion.
-
These two systems are passive transport systems because they do not require any additional energy to operate (however, facilitated diffusion requires ATP).
-
The major distinction between the two systems is the media in which they are used. Osmosis can only occur in a liquid medium, whereas diffusion can occur in all three (solid, liquid, and gas).
-
Furthermore, osmosis necessitates using a semipermeable membrane, whereas diffusion does not.
-
Water intake in plants is one example of osmosis. When a drop of food coloring is applied to a glass of water, the entire water content gets colored due to diffusion.
FAQs
1. What is the difference between diffusion and osmosis?
Diffusion may happen in any composition, including a semipermeable membrane, but osmosis always happens over a semipermeable membrane. One significant distinction between osmosis and diffusion is that both the solvent and solute particles are free to move in diffusion. Still, only the solvent molecules (water molecules) penetrate the barrier in osmosis.
2. What is the connection between diffusion and osmosis?
The relation between osmosis and diffusion is that the concentration of two liquids is equalized in both ways. Diffusion and osmosis are both passive transport processes, which means they do not require any additional energy input to proceed. In both diffusion and osmosis, particles travel from a high-concentration location to a low-concentration location.
3. What are examples of diffusion and osmosis?
Examples of Osmosis are:
- Absorption of water from the soil by plants
- Swelling of raisins or gummy sweets in water
- Movement of salt-water in animal cell
- Pruned fingers when exposed to water for longer periods. Skin absorbs water and expands.
Examples of diffusion that occurs in our daily life are.
- The smell of perfumes/Incense Sticks
- the CO2 diffuses in the air when Opening the Soda/Cold Drinks bottle
- tea bags in hot water.
- Dust particles or smoke diffused in the air, causing air pollution
- Breathing diffuses oxygen in our blood
- Transport Of Biomolecules and Minerals in Plants and Animals
- Sugar dissolves and sweetens the water.
- Waste Substances and Toxins being removed by kidneys from Our Body
4. What type of diffusion is osmosis?
Osmosis may be a subset of diffusion since it happens over a semipermeable membrane, and only water or another solvent flows. Diffusion and osmosis are passive transport mechanisms that equalize a solution’s concentration.
5. Is osmosis simple diffusion?
Yes, osmosis is simple diffusion.
6. What type of diffusion is diffusion?There are two forms of diffusion: simple diffusion and aided diffusion. Diffusion itself is an aided or facilitated diffusion.
7. How do you demonstrate osmosis and diffusion?
To demonstrate diffusion and osmosis in the context of cell biology, we shall zoom down to the molecular levels.
-
To demonstrate diffusion, we will drop some food coloring into a beaker of hot water and some food coloring into a beaker of cold water. We will observe that color spreads in hot water more rapidly than cold water. That’s because diffusion is based on molecular movement. Molecules tend to speed up at higher temperatures. Thus, diffusion happens more rapidly.
-
To demonstrate osmosis, we will use a thistle funnel tube. This tube has a length of a membrane stretched across the bottom. This is a semipermeable membrane. We will now fill the tube with a concentrate sucrose solution. Now suspend this tube in a beaker of distilled water. So, the solute concentration in the tube is much higher than that of water. We will observe that the water will osmose across the membrane and enter the tube.
8. What are the 2 types of diffusion?
Diffusion may be divided into simple diffusion and aided or facilitated diffusion.
We hope you enjoyed studying this lesson and learned something cool about Diffusion and Osmosis! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App and check out our awesome VR room for this guide – we promise it makes studying much more fun 😎
]]>