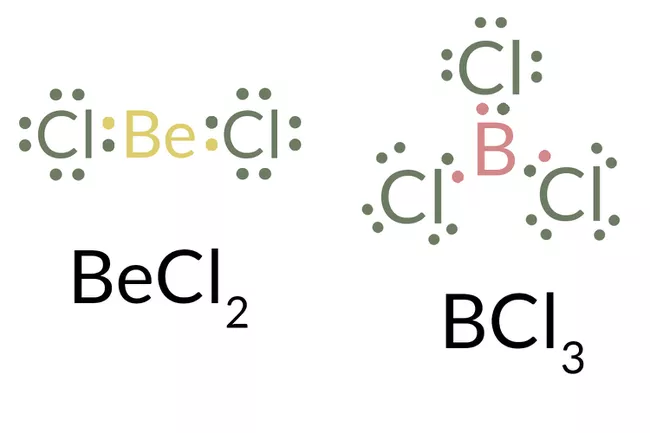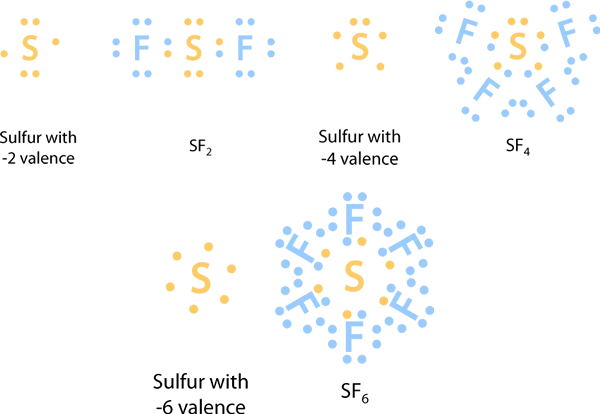Exceptions of the Octet Rule Study Guide
INTRODUCTION:
In the late 1940s, the phenomenon of nuclear fission was discovered. Nuclear fission is splitting an atom’s nucleus into two or smaller nuclei. At that time, the true potential of this phenomenon was not widely known. It was thought to be a process where a tremendous amount of energy was released, suitable only for annihilation.
Who would have thought that such a destructive form of energy could be used to light up our homes or street lights? The only exception is the difference in the environment where the reaction occurs. Like this exception, rules for molecule bonding also offer exceptions. Curious? Let’s know more regarding the types of exceptions to the octet rule and the list of elements that are exceptions to the octet rule!
WHAT IS THE OCTET RULE?
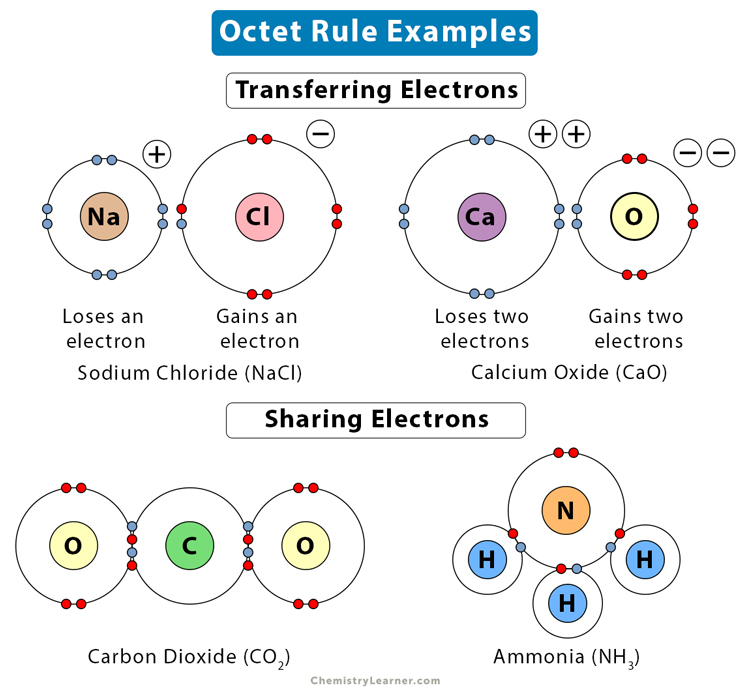
Before we look into the exceptions of the octet rule, we need to understand what the octet rule is! As per the octet rule, atoms gain or lose electrons in order to achieve an outer shell electron configuration that is closest to that of a noble gas. This is because the noble gas electronic configuration (eight-electron arrangement in the outer electron shell) is the most stable in the entire periodic table and it’s a natural tendency of any element to move towards more stability.
TYPES OF EXCEPTION TO OCTET RULE:
There are three types of exceptions to the octet rule:
(1) Incomplete octets
(2) Odd-electron molecules
(3) Enlarged octets
1. INCOMPLETE OCTET:
There aren’t enough electrons in hydrogen, beryllium, or boron to complete an octet. An octet is the eight-electron arrangement in the outer electron shell of the noble-gas atoms.
The core beryllium and boron atoms in the two molecules represented in this image have less than eight valence electrons and hence do not meet the octet rule.
2. EXPANDED OCTET:
Elements with periods larger than 3 have a d orbital with the same energy quantum number on the periodic chart. Although atoms in these periods obey the octet rule, there are times when their valence shells can extend to accept more than eight electrons.
This is most commonly seen with sulfur and phosphorus. As in the molecule SF₂, sulfur can follow the octet rule. Each atom has eight electrons around it. It is possible to sufficiently excite the sulfur atom to force valence electrons into the d orbital, allowing compounds like SF₄ and SF₆ to form. The sulfur atom in SF₄ contains 10 valence electrons, whereas the sulfur atom in SF₆ has 12 and hence do not meet the octet rule (8 valence electrons only).
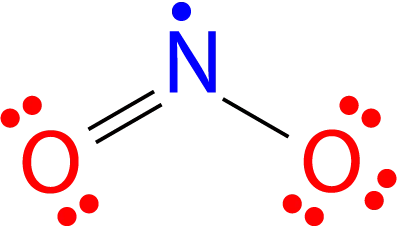
3. ODD ELECTRON SPECIES:
Pairs of electrons are found in the most stable compounds and complicated ions. There is a class of compounds in which the valence electrons in the valence shell have an odd number of electrons. Free radicals are the name for these molecules. One well-known example is nitrogen oxide (NO₂). Each oxygen atom contributes six valence electrons and the nitrogen atom contributes five for a total of seventeen. As the octet rule requires eight electrons around each atom, a molecule with an odd number of electrons must disobey the octet rule.
CONCLUSION:
- There are exceptions to the covalent bonding laws.
- These exceptions relate to atoms with electrons that won’t fit into the standard octet rule.
FAQs:
1. What elements can be an exception to the octet rule?
There aren’t enough electrons in hydrogen, beryllium, or boron to make an octet. There is only one valence electron in hydrogen, and there is only one site for it to make a connection with another atom. Beryllium only has two valence atoms and can only establish electron-pair bonds in two places.
2. What are the 3 exceptions to the octet rule?
The octet rule is subject to three basic exceptions: Molecules containing an odd number of electrons, such as NO; SF₆ molecules in which one or more atoms have more than eight electrons; and. Molecules contain more atoms with less than eight electrons, like BCl₃.
3. Is NH3 an exception to the octet rule?
Yes, of course, nitrogen contains 5 electrons in its outermost shell. It shares three electrons with each hydrogen atom and has one lone pair at the nitrogen atom, making it an excellent Lewis base.
We hope you enjoyed studying this lesson and learned something cool about the List of elements that are exceptions to the octet rule! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun, VR classrooms – we promise, it makes studying much more fun! 😎
SOURCES:
- Exception of octet. https://www.ck12.org/c/chemistry/exceptions-to-the-octet-rule/lesson/Exceptions-to-the-Octet-Rule-CHEM/. Accessed 26 Jan 2022.
- Incomplete octet- https://www.thoughtco.com/exceptions-to-the-octet-rule-603993. Accessed 26 jan 2022.
- Odd electron species- https://courses.lumenlearning.com/boundless-chemistry/chapter/exceptions-to-the-octet-rule/. Accessed 26 jan 2022.