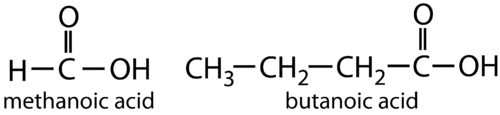Carboxylic Acids Study Guide
INTRODUCTION
Have you ever wondered how a carton of milk can go on without spoiling for a few days? Or how about a loaf of bread that stays for a week without spoiling? The answer is preservatives. But have you ever wondered what these preservatives are made up of?

Organic acids are quite well known for their excellent preservation capabilities. Their antibacterial activity stems from their capacity to switch from an undissociated to a dissociated state depending on the pH of the microenvironment, making them potent antimicrobial agents. Certain organic salts are employed as preservatives in milk and bread food products to avoid spoiling by preventing bacterial growth and fungal growth.
DEFINING CARBOXYLIC ACIDS
A carboxyl group is a group that consists of a carbonyl group linked to the very same carbon atom as a hydroxyl group. The organic acid formula for carboxyl groups is commonly expressed as -COOH or CO2H. Carboxylic acids are a kind of molecule defined by a single carboxyl group.
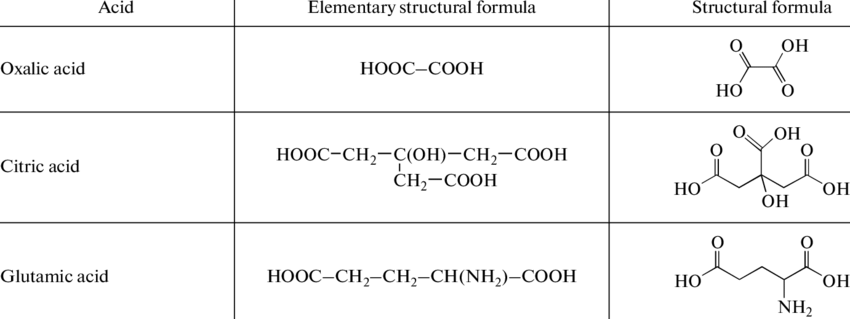
Here are a few examples of organic acid formula and structure:
Nomenclature of Carboxylic Acid
There are two systems to give the name to the organic compound which are common name and an IUPAC nomenclature. Depending on the source from which the carboxylic acid was obtained, the common term given to the acid varies.
Example: The word formic acid is derived from the substance known as formacia. In the case of acetic acid, the word acetum, which is present in vinegar, served as the inspiration for the name. This group’s additional carbon can be referred to as alpha carbon. Greek alphabets like alpha, beta, or gamma are used to denote the additional groups joined to the carboxylic acid.
Aliphatic Compound: The carboxylic acid is termed by substituting “-oic acid” for the prefix “-e” of the corresponding alkane. If there are many – COOH groups, “- e” is kept. The locant number one is always assigned to the carbon of the carboxylic group.
Aromatic Compounds: Aromatic carboxylic acids get their name from benzoic acid, which is its parent chemical. The suffix “carboxylic acid” is used to terminate IUPAC names.
Example: Since there is just one carbon in formic acid, we know it is methane. However, we should keep in mind that since – COOH is written as oic acid, methanoic acid will be printed instead. Methanoic acid is the IUPAC designation for formic acid. Similar to acetic acid, which has two carbons and must be expressed as ethanoic acid because we know it is ethane there. Acetic acid is known as ethanoic acid according to IUPAC.
ORGANIC ACID FORMULA OF METHANOIC ACID AND BUTANOIC ACID:
PROPERTIES OF CARBOXYLIC ACID
- Carboxylate ions are stabilized via resonance.
- Bronsted-Lowry acids include carboxylic acids that function as proton donors.
- The carbonyl group in carboxylic acids acts as a hydrogen bond acceptor, whereas the hydroxyl group acts as a hydrogen donor. As a result, they are frequently involved in hydrogen bonding.
- They have more stability and higher boiling temperatures than acid in an aqueous state because of their inclination to hydrogen bonds.
- Carboxylic acids are classified as weak acids in a neutral aqueous solution because they do not completely disintegrate to form H cations.
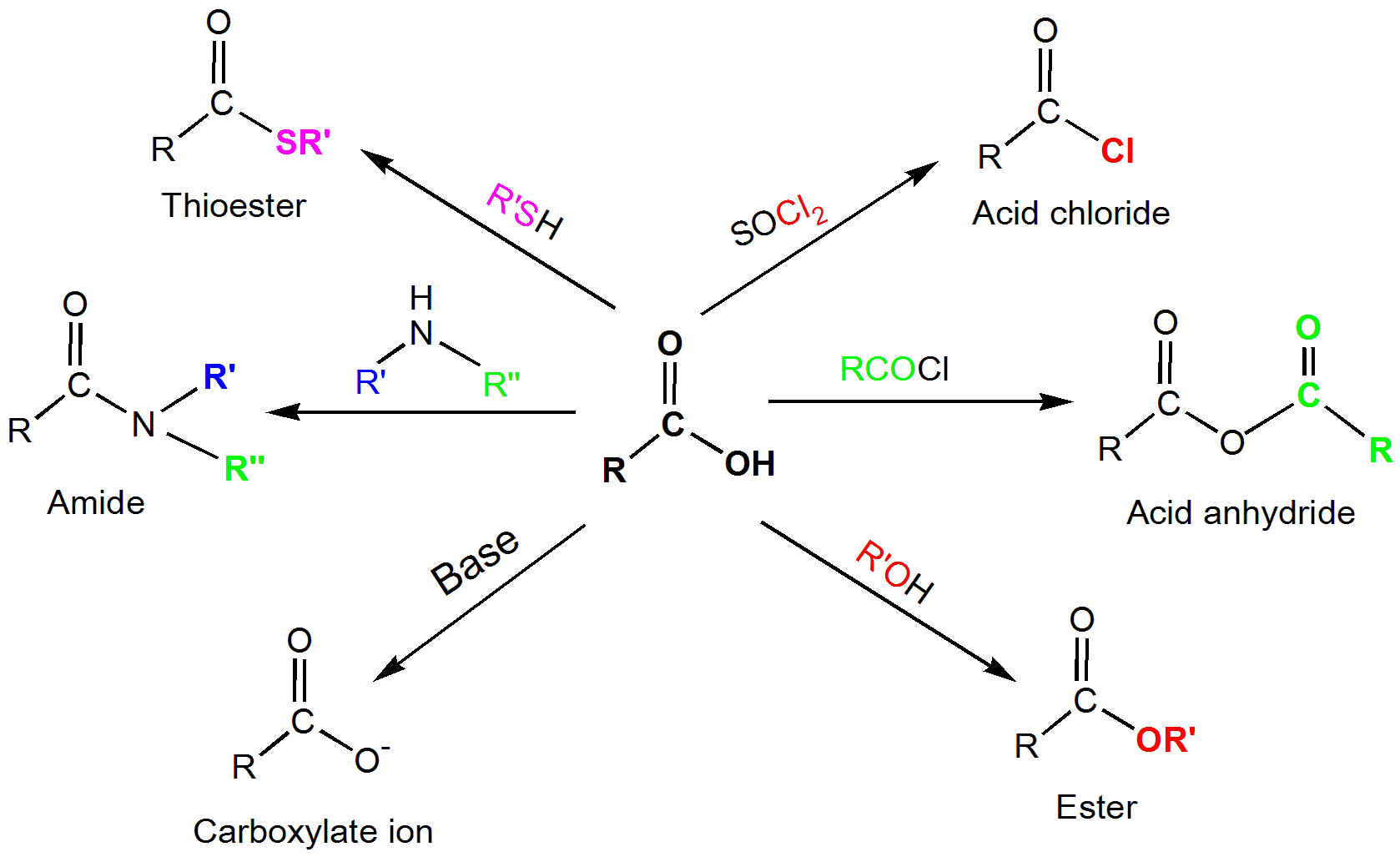
- The Schmidt reaction can be used to transform these compounds into amines.
- A carboxylic acid can be converted to alcohol by causing a hydrogenation reaction with hydrogen.
- These chemicals produce esters when they react with alcohol.
USES OF ORGANIC ACIDS
- Carboxylic acids are the building blocks of fatty acids necessary for human health.
- Soaps made with heavier fatty acids are also common.
- Many carboxylic acids are used to make soft drinks and other food items.
- These chemicals are used in the production of numerous medications. As a result, carboxylic acids play a crucial role in pharmacology.
- Compounds with the carboxyl functional group are used to manufacture various polymers.
CONCLUSION
- An organic substance with a carboxyl functional group is known as carboxylic acid.
- A carboxyl group is a group that consists of a carbonyl group linked to the very same carbon atom as a hydroxyl group.
- COOH is a common abbreviation for a carboxylic acid’s general formula.
FAQs
1. What are the examples of organic acid?
Lactic acid, acetic acid, and formic acid are just a few examples of organic acids.
2. Which acid is organic acid?
From formic acid until C18 acids, organic acids are aliphatic monobasic carboxylic acids.
3. What is the organic acid formula of oxalic acid?
C₂H₂O₄ is the formula of oxalic acid.
We hope you enjoyed studying this lesson and learned something cool about Carboxylic Acids! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun, VR classrooms – we promise, it makes studying much more fun! 😎
]]>