Straight-Chain Alkanes Study Guide
INTRODUCTION
Organic chemistry can be difficult but not impossible to understand. Before we get deep into the subject, we need to learn the basics of organic chemistry. So we need to understand what alkanes are? Alkanes are hydrocarbons; the compounds are made with hydrogen and carbons. Carbon is the 14th member of the periodic table; hence, it has a valency of 4.
WHAT ARE STRAIGHT-CHAIN ALKANES?
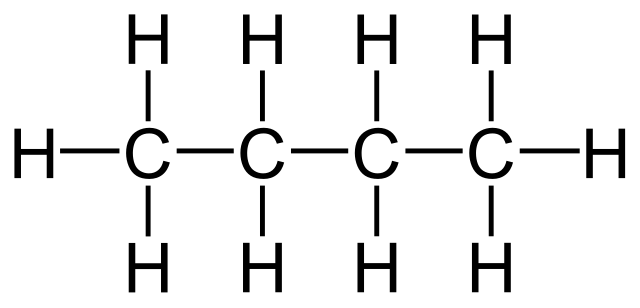
Straight chain alkanes are hydrocarbons, where the carbon atoms are connected in one continuous chain. There are no branches or sub-branches connected. You will find 2 carbon chains, 3 carbon chains to 10 carbon chains. The straight-chain alkanes help to understand the compounds and also ease the organic compound reactions.
HYDROCARBON CHAIN DEFINITION
A hydrocarbon chain can be defined as an organic molecule constituting carbon and hydrogen atoms organized in a chain. The carbon atoms are interconnected with the help of covalent bonding. And each carbon atom present in the chain is further bonded to 1 or even up to 3 hydrogen atoms. Some examples of hydrocarbons are methane, ethane, etc.
STEPS TO NAME STRAIGHT CHAIN ALKANES
Step 1: You need to identify the longest carbon chain that has a double bond.
Step 2: Then, you have to pick the prefix as per the name of the alkene established on the number of carbon atoms present in the chain.
Step 3: You need to number each carbon atom present in the longest carbon chain. This way, the double-bonded carbon atoms will have the lowest possible digit.
Step 4: Then, you have to determine the infix specified for the name of the given alkene as per the place of the double bond.
Step 5: Take note that the infix may not even be needed if the extended carbon chain contains only two or three carbon atoms.
Step 6: You need to determine the suffix for the name of the alkene.
Step 7: All consecutive chain alkenes that have one double bond will have “ene” at the end of their name.
Step 8: Then, you can write the name for the alkene according to prefix-infix-suffix.
FAQs
1. What are the first 10 straight-chain alkanes?
The first 10 Straight chain alkanes are as follows:
- Methane
- Ethane
- Propane
- Butane
- Pentane
- Hexane
- Heptane
- Octane
- Nonane
- Decane
2. How do you name a straight-chain alkane?
Naming a straight-chain alkane is very easy. The name of each alkane starts with a suffix that specifies the number of carbon atoms present and ends with a -ane. The molecular formula of the straight-chain alkane follows the pattern CₙH₂ₙ₊₂, where n is the number of carbon atoms present.
3. What is straight-chain alkane?
A straight-chain alkane is a hydrocarbon that only contains molecules of carbon and hydrogen atoms. Carbon has 4 valence electrons, and it is the 14th member of the periodic table. Hence, it can place only four atoms on four sides.
3. What is a straight-chain compound?
A straight-chain compound consists of carbon atoms connected in a continuous straight line. The carbon atoms are connected in a continuous chain; the carbon atom is bound to its neighbors, i.e., two carbon atoms and two hydrogen atoms.
We hope you enjoyed studying this lesson and learned something cool about Straight Chain Alkanes! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES
- IUPAC Naming Straight Chain Alkenes:https://www.ausetute.com.au/namsenes.html. Accessed 5th March 2022.
- Straight Chain Alkenes: https://chem.libretexts.org/Courses/University_of_Kentucky/UK%3A_CHE_103_-_Chemistry_for_Allied_Health_(Soult)/Chapters/Chapter_3%3A_Compounds/3.2%3A_Straight-Chain_Alkanes. Accessed 5th March 2022.