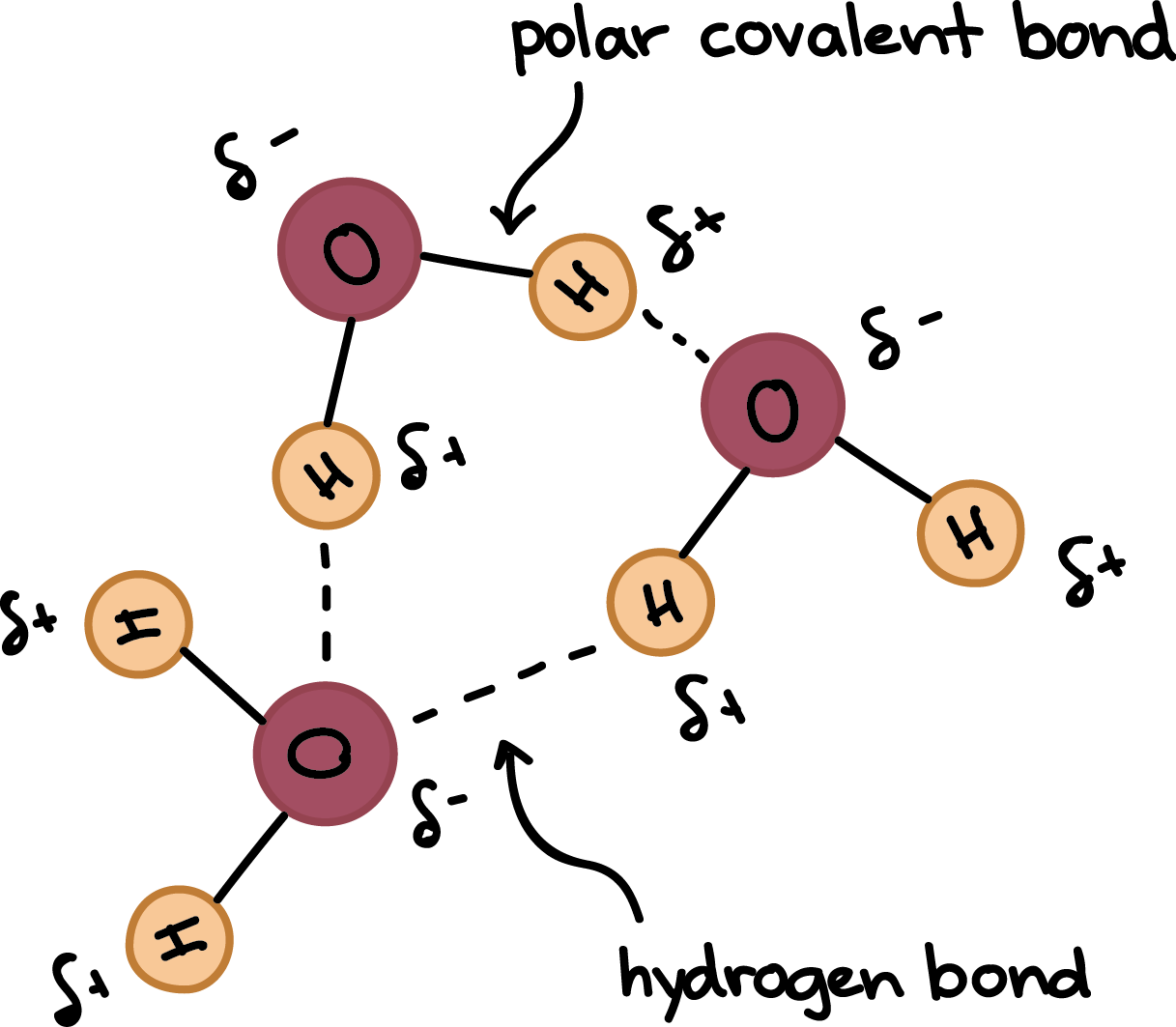Structure of Water and Hydrogen Bonding Study Guide
Introduction:
Water is indispensable to all forms of life. It possesses several remarkable chemical qualities that make it ideal for life support. In this article, let’s delve deeper into understanding water hydrogen bonding.
Solvent properties of water:
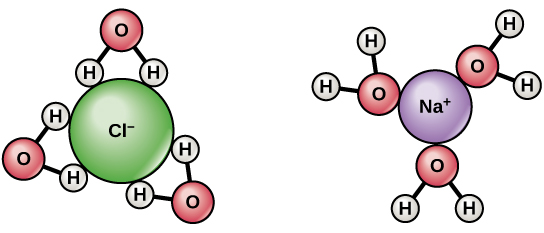
Water is frequently referred to as the “universal solvent” due to its ability to dissolve many solutes. However, some oils do not dissolve in water, and this is because water dissolves ions and polar molecules well but poorly dissolves nonpolar molecules. Water molecules are polar, having partial positive charges on the hydrogens, a partial negative charge on the oxygen, and a twisted overall structure.
Cohesion and adhesion of water:
- Water molecules can bind to each other, called cohesion, and other molecules called adhesion.
- Surface tension is caused by cohesive forces responsible for the tendency of a liquid’s surface to resist rupture when subjected to strain or stress.
- Spherical droplets are formed by water due to surface tension.
- Adhesion is the attraction of one type of molecule to another, and it may be relatively robust for water, especially when combined with other molecules of positive or negative charges.
Specific heat, the heat of vaporization, and density of water:
Water has a large specific heat capacity. It means that water can withstand a high amount of energy before its temperature is changed. Water also has a high heat of vaporization, which is the heat needed to move a compound from liquid state to gas state. The efficacy of evaporative cooling (why sweating makes one feel cooler) and the low density of ice (floating ice) are all explained by hydrogen bonding.
Water molecule polarity
The molecular structure of water explains that a water molecule comprises two hydrogen atoms connected to an oxygen atom, and its overall structure is curved. In addition to making bonds with the hydrogen atoms, the oxygen atom carries two unshared electrons.
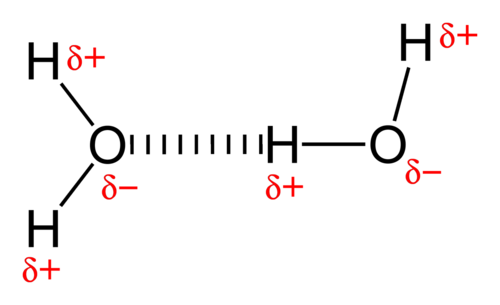
The shared as well as unshared electron pairs repel one another. Because oxygen is more electronegative (electron-greedy) than hydrogen, it grabs electrons and keeps them away from the H atoms. This results in a partial negative charge on the oxygen end of the water molecule and a partial positive charge on the hydrogen end. Thus water is categorized as a polar molecule.
Hydrogen bond
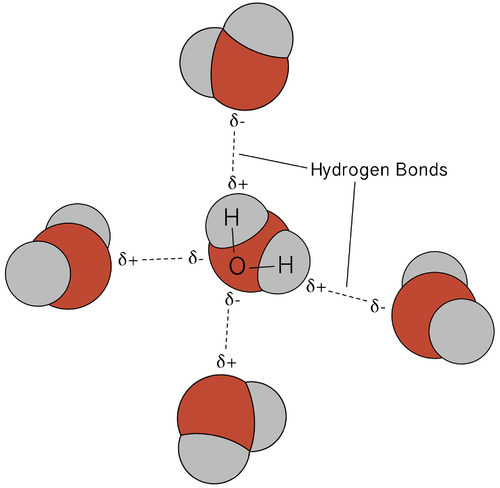
A hydrogen bond is an attractive intermolecular force in which a covalently bound hydrogen atom is attracted to a lone pair of electrons on an atom in a nearby molecule. Hydrogen bonds are quite strong compared to other dipole interactions, and a typicaL hydrogen bond has roughly 5% of the strength of a covalent bond.
Hydrogen bonding in the water molecules results in unique but highly significant features. Most molecular compounds with a mass similar to water are gases at room temperature. Water molecules can remain condensed in liquid due to strong hydrogen bonds. The diagram below depicts how the bent form and two hydrogen atoms per molecule enable each water molecule to bond to two other molecules.
- Water hydrogen bonds can break and regenerate when molecules travel from one location to another in the liquid state. The molecules of water begin to slow down as it cools.
- When water freezes to ice, the hydrogen bonds become permanent and create a highly specialized network.
- The distorted form of the molecules causes voids in the ice’s hydrogen bonding network.
- Ice has the odd virtue of being less dense in its solid form than in its liquid condition. In liquid water, ice floats.
Hydrogen bonding in water
A hydrogen bond is a dynamic attraction between surrounding water molecules that involves one hydrogen atom positioned between two oxygen atoms in water. In liquid water, hydrogen bonding occurs when the hydrogen atoms of one water molecule are attracted to the oxygen atom of an adjacent water molecule.
- Due to the component of polarity, water molecules attract each other.
- The positive end of one, a hydrogen atom, connects to an oxygen atom’s negative end. These are hydrogen bonds, weak connections that develop between a hydrogen atom with a partial positive charge and a more electronegative atom, such as oxygen.
- Hydrogen atoms in hydrogen bonding must be connected to electronegative atoms such as O, N, and F.
- Water molecules are attracted to other polar molecules as well as ions. Hydrophilic refers to a charged or polar material that interacts with and dissolves in water: hydro means “water,” and philic means “loving.”
- Oils and fats, for example, are nonpolar compounds that do not interact well with water. They are hydrophobic (phobic means “fearful”) because they separate from it rather than dissolve in it.
- The pH scale runs from 0 to 14 and is a measur of how acidic or basic water is — acidic (pH < 7), neutral (pH = 7), alkaline/basic (pH >7).
- An acid is any substance that reacts to liberate hydrogen ions in water solution. In contrast, a base accepts hydrogen ions. Pure water has a pH of 7 and is considered “neutral” because it has neither acidic nor basic properties.
Conclusion:
- The water molecule’s polarity is important in the dissolution of ionic substances during aqueous solution production.
- A hydrogen bond is a force in which a hydrogen atom that is covalently bonded to a minute, extremely electronegative atom is attracted to a lone pair of electrons on an atom in an adjacent molecule.
FAQs:
1. How do hydrogen bonds affect the structure of water?
The hydrogen bonds affect the solid structure of water; as water freezes, the bonds become more stiff, giving ice a more open and less dense overall structure. The existence of hydrogen bonding also makes water molecules more’ sticky,’ or in scientific language, cohesive and adhesive.
2. What will happen if hydrogen bonding in water does not exist?
Without hydrogen bonding, water molecules would move more quickly and with less thermal heat input, causing the temperature to rise higher for each calorie of heat supplied. This would also significantly lower the heat energy required for phase transitions from ice to liquid and liquid to vapour.
3. What causes hydrogen bonding in water?
In the particular case of water, hydrogen bonds develop between adjoining hydrogen and oxygen atoms of adjacent water molecules.
4. What is the most important role of hydrogen bonding between water molecules?
Water molecules can remain condensed in liquid due to strong hydrogen bonds.
5. What are the conditions necessary for forming a hydrogen bond?
Hydrogen bonding has two prerequisites:
(i). The first molecule has a hydrogen bond to a strongly electronegative atom (N, O, F).
(ii). A lone pair of electrons on a minute, extremely electronegative atom characterizes the second molecule (N, O, F).
6. How do you break hydrogen bonds in water?
These linkages can be broken by simply adding another material or substance to the water.
We hope you enjoyed studying this lesson and learned something cool about the Structure of Water and Hydrogen Bonding! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
Sources:
- Water. https://www.britannica.com/science/water. Accessed on 16 Dec, 2021.
- Structure of Water. https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_Introductory_Chemistry_(CK-12)/15%3A_Water/15.01%3A_Structure_of_Water. Accessed on 16 Dec, 2021.
- The Structure of Water. https://healingearth.ijep.net/water/structure-water. Accessed on 16 Dec, 2021.
- AP Biology Course and Exam Description https://apcentral.collegeboard.org/pdf/ap-biology-course-and-exam-description-0.pdf?course=ap-biology Accessed on 16 Dec, 2021.