Le Chatelier’s Principle Study Guide
INTRODUCTION
Have you ever experienced an upset stomach after eating spicy food? The discomfort we feel after enjoying a good meal of hot wings or spicy curry comes from the acid-inducing properties of the spices they contain.
The increased production of concentrated hydrochloric acid within the lining of the stomach causes acid reflux. There are several antacids available in the market that tend to suppress acid production and return the status quo. However, these medicines can do more harm than good because of their unnatural chemical composition.
This is when a proven home remedy comes into play. You can take a tablespoon of baking soda and mix it in water. Drinking this solution will give you relief immediately. The science behind this hack is Le Chatelier’s principle of chemical equilibrium. Baking soda and antacids are common examples of the effect of change of concentration on reaction rate. Let’s talk about how.
LE CHATELIER’S PRINCIPLE
In 1884, Henri Louis Le Chatelier proposed that changes in the temperature, pressure, or concentration of a substance in a chemical reaction causes an opposing reaction in the system. This opposing reaction is supposed to drive the system into a new state of equilibrium by increasing the concentration rate in the opposing direction.
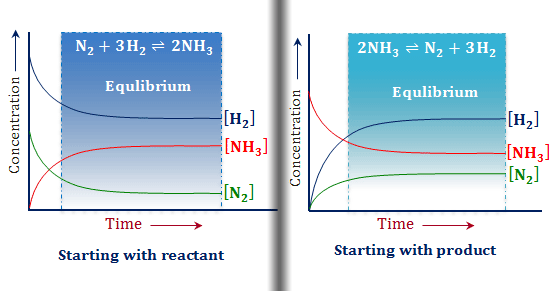
The equilibrium state is said to be achieved when the rate of forwarding and reverse reaction becomes equal. The industrial application of this principle includes manipulating the concentration of reactants or products to increase or decrease the production of the desired substance.
EFFECT OF CONCENTRATION ON RATE OF REACTION
Le Chatelier’s principle suggests that reversible reactions are self-correcting. This implies that reversible chemical equations always move in a direction where they can achieve a new equilibrium state.
Therefore, if we increase the quantity of reactants or decrease the quantity of the products, the reaction will shift to the right until the rate of forwarding and the reverse reaction is the same. Let’s look at an example for a better understanding:
CO + 2H₂ ⇌ CH₃OH
In this equation, the double arrow signifies that the reaction moves in both the forward and backward direction. If we increase the concentration of carbon monoxide, the production of methanol will increase according to Le Chatelier’s principle. The purpose would be to counter the increase in CO in the system and return to the equilibrium state.
CONCLUSION
- Le Chatelier’s principle states that when the equilibrium of a reaction is disturbed by a change in pressure, temperature, or solute concentration, an opposing reaction takes place that shifts the position of the equilibrium to produce a different ratio of products.
- if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to counteract the change to reestablish an equilibrium
- Chemical equilibrium is a state in a chemical reaction when the forward and backward reaction rates in a reversible reaction become equal.
- When we increase the concentration of reactants or decrease the concentration of products in a reversible reaction, the concentration of products increases according to Le Chatelier’s principle.
FAQs
1. What is the effect of concentration change?
According to Le Chatelier’s principle, if we increase the concentration of reactants or products, the rate of reaction will increase to the opposite side to achieve a new equilibrium state.
2. What is the effect of higher concentration?
Higher concentration in a reversible chemical reaction will shift the equilibrium to the opposite side to counter the extra quantity in the system.
We hope you enjoyed studying this lesson and learned something cool about Le Chatelier’s Principle! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise, it makes studying much more fun! 😎
SOURCES
Le Chatelier’s Principle: https://courses.lumenlearning.com/introchem/chapter/le-chateliers-principle/#:~:text=According%20to%20Le%20Chatelier’s%20principle,shift%20equilibrium%20to%20the%20right.Accessed 9th March 2022. Watch it Fizz: https://www.ck12.org/c/chemistry/effect-of-concentration/rwa/Watch-It-Fizz/.Accessed 9th March 2022.
]]>