Physical Properties Study Guide
INTRODUCTION
Suppose you took 250 mL of water and poured it into a container, and then poured the same water into another container. Did the volume of water change, or did it remain the same when you poured it into a different container?Volume is a physical property and is why it did not change when the water was poured into two different containers. Let’s understand what physical properties are.
PHYSICAL PROPERTY
Any property that can be measured without changing the chemical identity of a substance is its physical property. For example, when we pour water in two different containers, it will remain water only. That is, its properties are not changing while we are measuring the volume. Some of the physical properties are volume, color, hardness, malleability, solubility, electrical conductance, density, melting, and boiling points. Let’s understand what these properties are!
COLOR
Blood is red in color, while a leaf is green. We can see and measure the greenness of a leaf without changing its identity. That is why it is a physical property. Mostly all the elements in the periodic table are silver, gray, or do not have color. Some of the elements do have color, as chlorine is yellow.
HARDNESS
Hardness is determined by how hard a substance is. Generally, metals are hard such as copper, tungsten, titanium, etc., but there are metals that can be cut with a knife like sodium and potassium.
MALLEABILITY
Property of a substance by which it can be beaten into thin sheets. For example, we use aluminum foils to wrap our food because aluminum is malleable and can be beaten into thin sheets.
MELTING AND BOILING POINT
The temperature at which a substance melts is called a melting point and at which it boils, which is called a boiling point. Melting and boiling points are used to identify the substance as well as to find its purity.
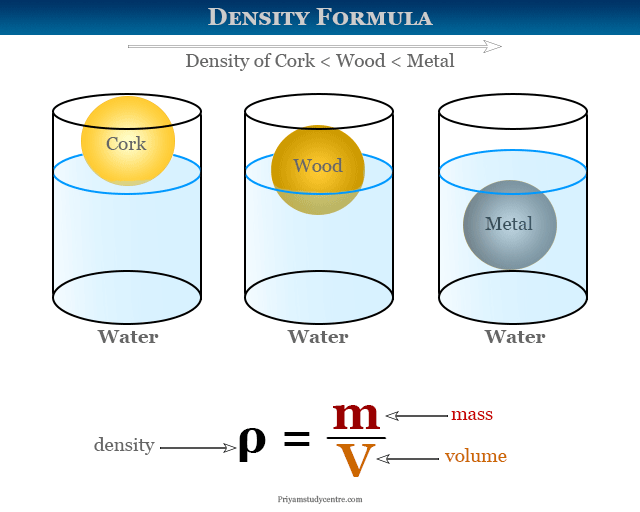
DENSITY
Density is how heavy a substance is in comparison to its size or how compact a substance is. Osmium and iridium are the densest metals in the periodic table, while hydrogen gas is the least dense.
Density is given by the formula: Mass/ Volume.
- Any substance which is less dense than water floats on it, for example, cork in this case.
- Any substance which is denser than water sinks. For example, when metal is put in water, it sinks.
- Density is also used to identify an element.
CONCLUSION
- Physical properties are those which can be measured without changing the identity of a substance.
- Color, hardness, volume, density, malleability, solubility, boiling, and melting points are some of the physical properties.
FAQs
1. Is beating substance into thin sheets, i.e., malleability, a physical property?
Yes, malleability is a physical property as by beating a substance into thin sheets, the substance’s identity is not changing.
2. Is aluminum less or more dense than water?
Aluminum is denser than water; that is why it settles at the bottom of a beaker filled with water.
3. What is the formula of density?
Density = Mass/ Volume
We hope you enjoyed studying this lesson and learned something cool about Physical Properties! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES
- Physical Properties: https://www.teachoo.com/12468/3425/Question-1-Page-6/category/Questions-from-Inside-the-chapter/. Accessed 28 Feb 2022.
- Physical properties: https://www.ck12.org/c/chemistry/physical-properties/lesson/Physical-Properties-CHEM/ Accessed 28 Feb 2022.