Radioactivity Study Guide
INTRODUCTION
On the 6th of August 1945, American forces launched an atomic bomb on Hiroshima, Japan. It killed nearly 90,000 to 120,000 individuals, who perished from injuries or severe radiation illness either immediately or during the coming weeks and months. The aftermath of radiation, which caused more significant issues such as damage to bone marrow and the digestive tract, set this explosion apart from other gunpowder bombs. In this article, we will study the radioactive properties of elements.
WHAT IS RADIOACTIVITY
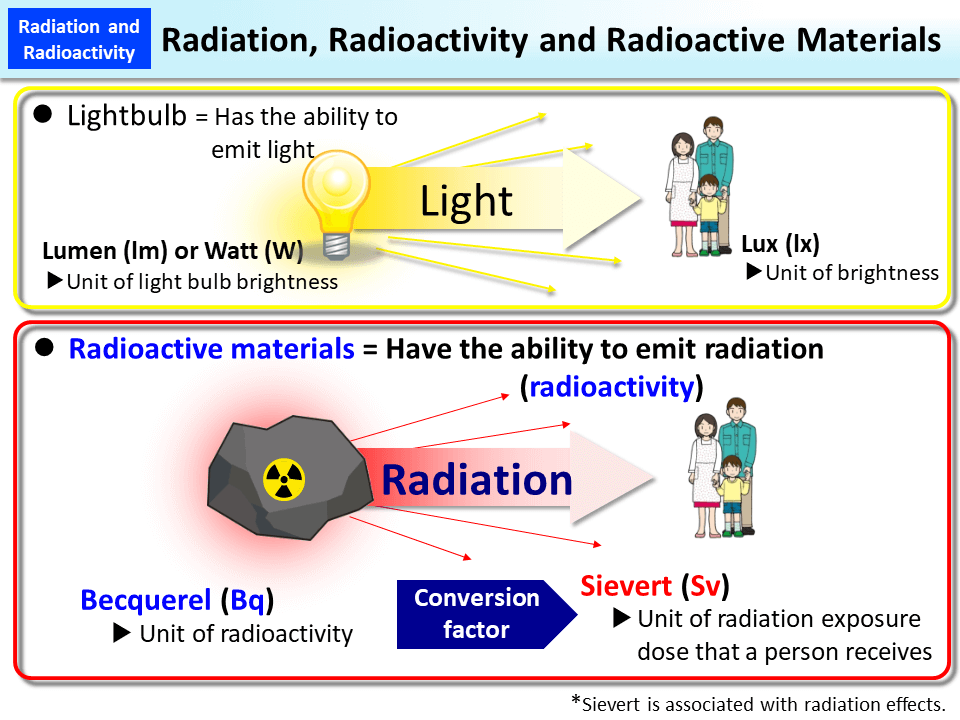
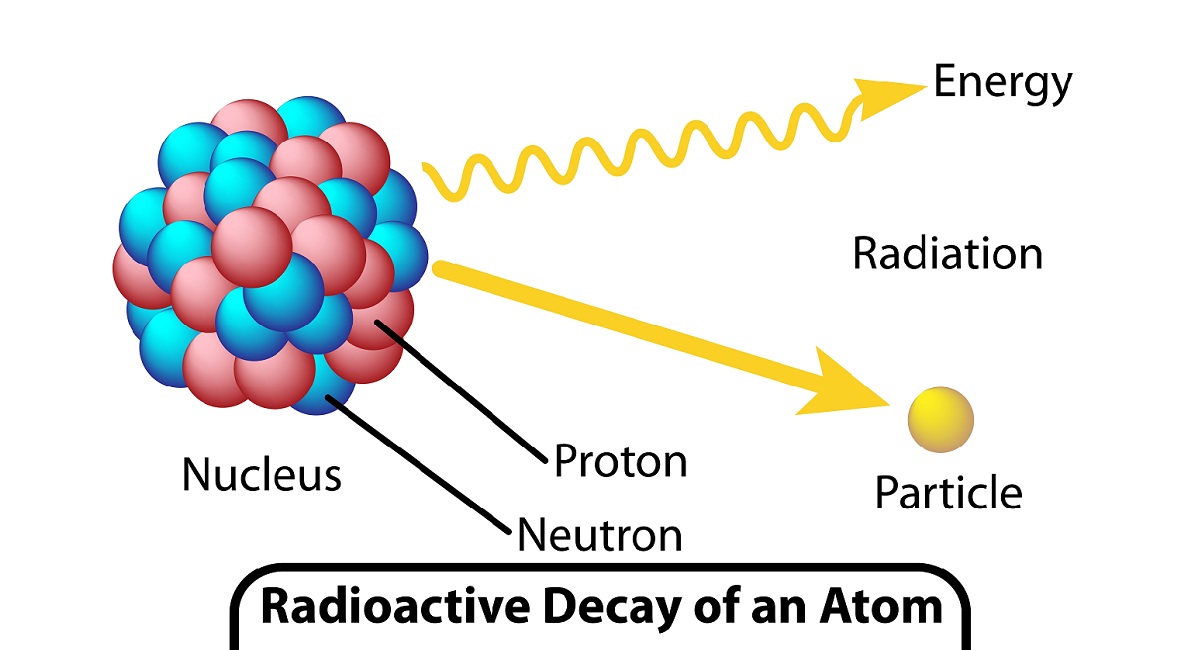
- The spontaneous emission of radiation in the form of particulate or high-energy photons due to a nuclear process is known as radioactivity.
- Radioactive decay, nuclear deterioration, nuclear disintegration, and radioactive disintegration are all terms for the same thing.
- While there are various types of electromagnetic radiation, radioactivity is not usually the source. A light bulb, for example, emits radiation in the form of heat and light but is not radioactive.
- The nucleus of an atom shows radioactivity as a result of nuclear instability. Radiation released from an atom’s unstable nucleus causes energy to be dissipated.
UNCERTAINTY IN RADIOACTIVE DECAY
- At the level of individual atoms, radioactive decay is a randomized or stochastic phenomenon.
- While it is difficult to forecast when a solitary unstable nucleus would decay, decay rates or half-lives may be used to estimate the rate of disintegration of a collection of atoms.
- A half-life is the amount of time it takes for half of a sample of matter to decay radioactively.
LAW OF RADIOACTIVITY
- The disintegration of the nucleus produces radioactivity.
- The rule of conservation of charge governs radioactivity.
- The offspring nucleus has distinct physical and chemical characteristics than the mother nucleus.
- Alpha, beta, and gamma particles are always present when energy is emitted by radioactivity.
- The number of atoms present at the moment determines the rate of decay of radioactive compounds.
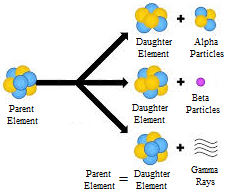
TYPES OF RADIOACTIVE DECAY
Alpha, beta, and gamma decay were the first three forms of radioactive decay known. The capacity to permeate matter gave these decay types their names. The shortest distance is covered by alpha decay, whereas the longest distance is covered by gamma decay.
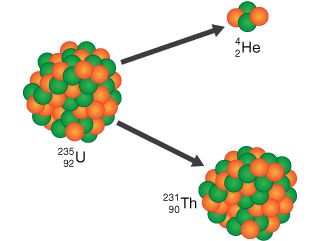
ALPHA DECAY:
A nucleus splits up into two pieces in alpha decay: a pair of protons coupled to a pair of neutrons (a cluster of four particles that is effectively a helium nucleus and is termed an alpha particle), and the parent nucleus minus alpha particle.
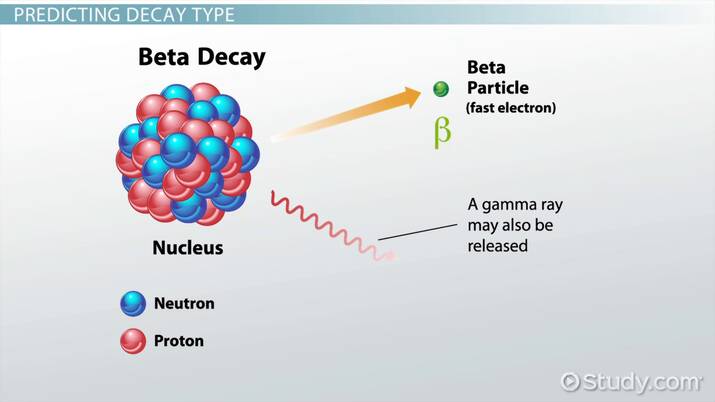
BETA DECAY:
- One of the neutrons in the nucleus turns into a proton via beta decay, resulting in a rise in the atomic number of the element.
- The nucleus not only changes its atomic number but also develops and discharges an electron from the atom to balance out the positive charge it receives by converting a neutron to a proton.
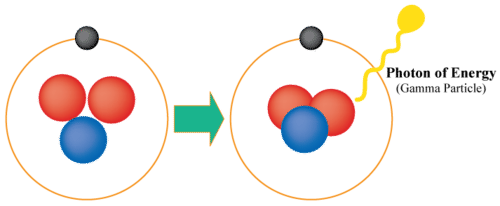
GAMMA DECAY:
- The nucleus emits radiation without altering its makeup during gamma decay. Because photons may penetrate through most typical shielding materials and induce DNA damage in living cells, gamma rays are among the most harmful forms of radiation.
- But this could be used in beneficial ways as well like gamma rays are used in radiotherapy to destroy malignant cells. They’re also utilized to scan the insides of the body.
CONCLUSION
- Radioactive decay occurs when a nucleus spontaneously loses some of its mass and emits radiation.
- The alpha, beta, and gamma radioactive decay products will be discussed here in order of their capacity to permeate materials.
FAQs
1. What is radioactivity in simple words?
Radioactivity is the act of producing radiation autonomously, as the term indicates. This is accomplished by an unstable atomic nucleus that “desires” to lose some energy in order to move to a more stable form.
2. What is radioactivity and what are the different types?
Contingent on what particles or energy are emitted during the reaction, multiple forms of radioactivity exist. Alpha particles, beta particles, and gamma rays are the three most frequent forms of radiation.
We hope you enjoyed studying this lesson and learned something cool about Radioactivity! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise, it makes studying much more fun! 😎
SOURCES
- Radioactivity: https://chem.libretexts.org/Courses/University_of_Missouri/MU%3A__1330H_(Keller)/21%3A_Nuclear_Chemistry/21.1%3A_Radioactivity . Accessed 14 march 2022.
- Definition of Radioactivity: https://www.thoughtco.com/definition-of-radioactivity-606338. Accessed 14 march 2022.
- Radioactivity Decay Types: https://www.khanacademy.org/science/in-in-class-12th-physics-india/nuclei/in-in-nuclear-physics/a/radioactive-decay-types-article. Accessed 14 march 2022.