Reactants, Products, and Leftovers Study Guide
INTRODUCTION
Over the decades, several methods of documenting recipes have evolved. Cookbooks are likely to be written by people who gathered all of their own recipes. Cookbooks in paperback become accessible later. We can now discover recipes on a variety of websites and rapidly seek information about how to prepare almost anything. Although understanding a few codes and symbols (what’s the distinction between a tsp and a Tsp?) is occasionally required when reading a recipe, knowing what we begin with and what we eventually wind up with is always present.
A similar case is that of a chemical reaction. Starting with reactants and ending up with products, the knowledge about the reaction is composed in the form of a chemical reaction.
CHEMICAL REACTION
A chemical equation can be used to illustrate the interaction between zinc and sulfur. In terms, the reaction may be described as follows:
Zinc + Sulfur → Zinc sulfide
The symbols and formulae of the chemicals involved are a more convenient method to represent a chemical reaction:
Zn + S → ZnS
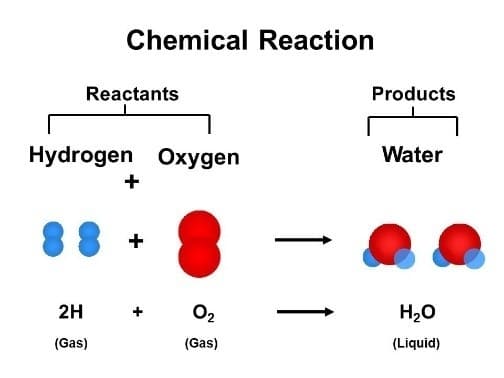
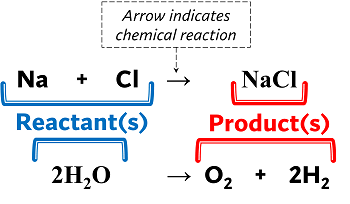
Reactants are the substances towards the left side of the arrow in an equation. A reactant is a material that is available when a chemical reaction begins.
Products are the material(s) towards the right of the arrow. A product is a material that remains after a chemical reaction has been completed. Zinc and sulfur are the reactants in the equation given, and they mix chemically to generate the zinc sulfide product.
Excess reagents, also referred to as leftovers, in a chemical reaction are reactants that haven’t been used completely when the reaction is completed. Because its quantity restricts the number of products generated, the limiting reagent is the one that is totally used up or reacted.
Chemistry strives to keep leftovers and pocket pieces to a minimum. We can’t make or destroy stuff in typical chemical reactions (law of conservation of mass). For instance, we must finish up with 10 carbon atoms if we begin with 10 carbon atoms. That’s when the balancing of chemical reactions comes into play.
CONCLUSION
- A chemical equation is an empirical representation of a chemical process.
- A reactant is a material that is available when a chemical reaction begins.
- A product is a material that remains after a chemical reaction has been completed.
FAQs:
1. What are the leftover reactants called?
Excess reagents are reactants that have not been used up when a chemical reaction is complete.
2. What are reactants and products?
Reactants are the substances towards the left side of the arrow in an equation. A reactant is a material that is available when a chemical reaction begins. Products are the material(s) towards the right of the arrow. A product is a material that remains after a chemical reaction has been completed.
We hope you enjoyed studying this lesson and learned something cool about Reactants, products, and leftovers! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES:
- Inorganic Chemistry: https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry Accessed 5 Feb 2022.
- Chemical Reactions: https://courses.lumenlearning.com/cheminter/chapter/chemical-reactions/ Accessed 5 Feb 2022.
- Reactants and Products: https://courses.lumenlearning.com/cheminter/chapter/reactants-and-products/ Accessed 5 Feb 2022.