CBSE Class 10 Science Chapter 3 Revision Notes
Chapter 3: Metals and Non- metals Revision Notes
Alloys
- Alloys are homogenous metal-to-metal or metal-to-nonmetal combinations. Alloy formation improves the material’s desired qualities, such as hardness, tensile strength, and corrosion resistance.
A few examples of alloys:
Brass is made up of copper and zinc.
Bronze is made up of copper and tin.
Lead and tin solder
Amalgam is a mixture of mercury and other metals.
Corrosion
- The action of moisture, air, or chemicals in the surrounding environment causes gradual degradation of a substance, generally a metal.
- Rusting:
4Fe(s)+3O2(from air)+xH2O(moisture)→2Fe2O3. xH2O(rust)
- Copper corrosion is a type of corrosion that occurs when copper is exposed to air.
Cu(s)+H2O(moisture)+CO2(from air)→CuCO
- Silver corrosion is a term used to describe the process of a metal corroding.
Ag(s)+H2S(from air)→Ag2S(black)+H2(g)
Prevention of Corrosion
-
Painting, oiling, or greasing metal surfaces: Painting, oiling, or greasing metal surfaces keeps air and moisture out.
-
Alloying: Alloyed metal is more corrosion resistant. Take, for example, stainless steel.
-
Galvanization: This is the process of coating iron items with molten zinc. Zinc inhibits corrosion by forming a protective coating.
-
Electroplating: Electroplating is a technique for coating one metal with another using an electric current. This procedure not only protects the metal, but it also improves its beauty.
Silver plating and nickel plating are two examples.
- Magnesium is more reactive than iron, hence it serves as a sacrificial shield. When coated on iron or steel items, it works as a cathode, undergoing reaction (sacrifice) in place of iron and protecting the materials.
PHYSICAL PROPERTIES
Physical Properties of Metals
● Hard and have a high tensile strength
● Solid at room temperature
● Sonorous
● Good conductors of heat and electricity
● Malleable, i.e., can be beaten into thin sheets
● Ductile, i.e., can be drawn into thin wires
● High melting and boiling points (except Caesium (Cs) and Gallium (Ga))
● Dense, (except alkali metals). Osmium – highest density and lithium – least density
● Lustrous
● Silver-grey in colour, (except gold and copper)
Non-Metals
Nonmetals are elements that don’t have the same characteristics as metals.
Physical Properties of Non- Metals
- At room temperature, they exist as solids, liquids, and gases.
- Brittle
- Non-malleable
- Non-ductile
- Non-sonorous
- Heat and electricity conductors that aren’t up to par
Physical Properties with Exceptions
- Knives can be used to cut alkali metals (Na, K, and Li).
- Mercury is a metal that is liquid at room temperature.
- Heat conductivity is weak in lead and mercury.
- The slightest change in temperature causes mercury to expand dramatically.
- The melting points of gallium and caesium are quite low.
- Iodine is a non-metallic substance with a lustrous sheen.
- Graphite is a material that conducts electricity.
- Diamond is a heat conductor with a high melting point.
CHEMICAL PROPERTIES
Chemical Properties of Metals
- Alkali metals (Li, Na, K, and so on) have a strong reaction with water and oxygen or air.
- When Mg comes into contact with hot water, it reacts.
- Steam reacts with Al, Fe, and Zn.
- Water and dilute acids do not react with Cu, Ag, Pt, or Au.
Reaction of Metals with Oxygen (Burnt in Air)
- Metal + Oxygen Metal oxide is a combination of metal and oxygen (basic)
- Because Na and K react violently with air and catch fire, they are maintained submerged in kerosene oil.
4K(s)+O2(g)→2K2O(s) (vigorous reaction)
Mg, Al, Zn, and Pb react slowly with air and produce a corrosion-resistant coating.
2Mg(s)+O2(g)→2MgO(s) (Mg burns with white dazzling light)
4Al(s)+3O2(g)→2Al2O3(s)
Silver, platinum, and gold are inert metals that do not burn or react with air.
Basic Oxides of Metals
- Alkalis are formed when metallic oxides dissolve in water. Their aqueous solution changes from red to blue litmus.
Na2O(s)+H2O(l)→2NaOH(aq)
K2O(s)+H2O(l)→2KOH(aq)
Metal Amphoteric Oxides
- Amphoteric oxides are metal oxides that create salt and water when they react with acids and bases.
Al2O3, ZnO, PbO, and SnO are other examples.
Al2O3(s)+6HCl(aq)→2AlCl3(aq)+3H2O(l)
Al2O3(s)+2NaOH(aq)→2NaAlO2(aq)+H2O(l)
ZnO(s)+2HCl(aq)→ZnCl2(aq)+H2O(l)
ZnO(s)+2NaOH(aq)→Na2ZnO2(aq)+H2O(l)
EXTRACTION OF METALS
Manufacturing of steel
Thermite reaction: Al(s)+Fe2O3(s) → Al2O3+Fe(molten)
Welding of railway tracks, fractured machine components, and other applications employ the thermite reaction.
Metals’ Occurrence
- The majority of elements, particularly metals, exist in nature in combination with other elements. Minerals are made up of all of these metal compounds. However, only a handful of them are viable suppliers of that metal. Ores are the name for such sources.
- Au, Pt – can be found in their natural or unprocessed condition.
- Ore enrichment is a term used to describe the process of enriching ore.
- It refers to the physical and chemical techniques used to remove impurities or gangue from ore. The process utilised for a certain ore is determined by the differences in the ore’s and gangue’s qualities.
Metal Refining is a process that involves the refinement of metals.
Metal refining is the process of eliminating impurities and gangue from raw metal. It is the final phase in metallurgy and is based on the differences in metal and gangue characteristics.
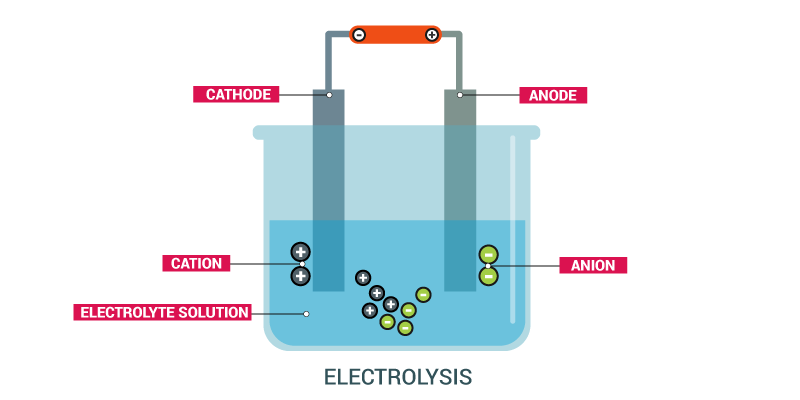
Refining by Electrolysis
- Copper, zinc, nickel, silver, tin, gold, and other metals are purified electrolytically.
- Impure or unrefined metal is used as an anode.
- A thin strip of pure metal is used as a cathode.
- Aqueous solution of metal salts is used as an electrolyte.
- Metal ions are discharged into the solution from the anode (oxidation).
- The corresponding quantity of metal from the solution is deposited at the cathode (reduction).
- Impurities collect near the anode’s bottom.
- Compounds with an ionic charge
- The complex is held together by electrostatic interaction between the oppositely charged ions.
- MgCl2, CaO, MgO, NaCl, and other salts are examples.
Ionic Compound Properties
- Usually, they’re crystalline solids (made of ions).
- Melting and boiling points are quite high.
- When melted and in aqueous solution, they conduct electricity.
- Water and polar solvents are the most common solubilizers.
Ionic Compounds and Electric Conduction
- When ions become free and function as charge carriers in a molten or watery state, ionic compounds transmit electricity.
- Ions in solid form are kept in place by electrostatic forces of attraction and are thus unable to conduct electricity since they are not free to move.