CBSE Class 12 Chemistry Chapter 14 Revision Notes
Chapter 14: Biomolecules Revision Notes
Carbohydrates
- Carbohydrates are a group of compounds that includes polyhydric aldehydes and ketones, as well as a wide range of other polymeric molecules that produce these when hydrolyzed, such as sugars, glycogen, cellulose, starch, and so on.
- Carbohydrates are classified into three groups based on how they react to hydrolysis: Monosaccharides (glucose, fructose, and other sugars), disaccharides (sucrose, maltose, and other sugars), and polysaccharides (e.g., starch, cellulose, etc.)
- Carbohydrates can also be divided into sugars and non-sugars. Sugars have a sweet taste, are crystalline, and are water soluble. Mono and oligosaccharides are the most common sugars. Non-sugars are flavorless, amorphous, water insoluble, and primarily made up of polysaccharides.
- Sugars can be classified as reducing or non-reducing carbohydrates. Tollens’ and Fehling’s solution tests are used to detect the presence of reducing sugars. This includes all monosaccharides, aldoses, and ketoses. Other oligosaccharides may be decreasing as well.
- Non-reducing carbohydrates encompass all polysaccharides (starch, cellulose, glycogen etc). Sucrose is a non-reducing disaccharide sugar.
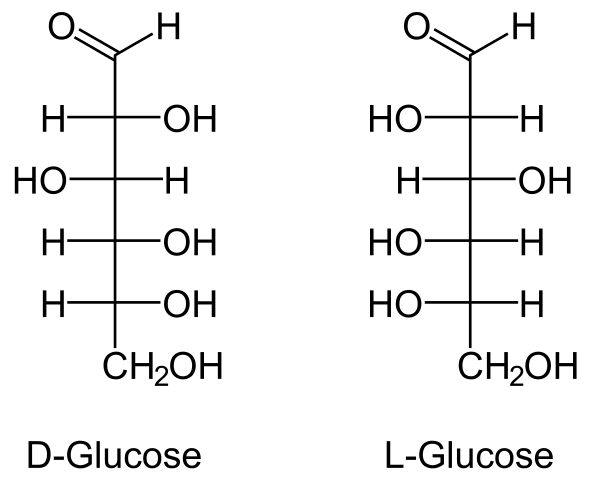
- Glucose’s molecular formula is C6H12O6. It’s made by boiling sucrose in alcoholic solution with dil HCl or dil H2SO4 or by hydrolyzing starch with dil H2SO4 at 393 K under pressure.
- Anomers are a pair of stereoisomeric ring forms of any sugar that differ only in configuration at carbon 1 (the anomeric carbon).
- Mutarotation is the spontaneous change in specific rotation of an optically active sugar when it is dissolved in water.
- R. D. Haworth proposed the cyclic structure of glucose. The pyranose structure (α or β) is a six-membered cyclic structure of glucose.
- When sucrose is hydrolyzed, the sign of rotation changes from dextro (+) to laevo (-). Sugar inversion is a term used to describe such a change.
- Amylum (C6H10O5)n is a D-glucose polymer made up of two components: amylose and amylopectin. Amylose makes up 15–20 percent of natural starch, while amylopectin makes up 80–85 percent.
- Cellulose (C6H10O5)n is a -D-glucose linear polymer in which the β-D-glycosidic linkage connects the β-D-glucose units.
Proteins
- Proteins are the most common biomolecules in living systems. They are necessary for body growth and maintenance. Polymers of α-amino acids make up all proteins.
- Amino acids are organic compounds with at least one amino (-NH2) and one carboxyl (-COOH) functional group. Depending on the position of the two functional groups in the alkyl chain, amino acids are classified as α , β , γ, δ and so on.
- In the proteins of living things, there are 20 different amino acids, each with a different – R group. Ten of the twenty amino acids found in proteins can be synthesized by the human body. These non-essential amino acids include glycine, alanine, aspartic acid, glutamic acid, and others.
- Human beings require certain amino acids for proper health and growth. However, the human body is unable to produce them. These essential amino acids, such as valine, leucine, and phenylalanine, must be supplied to the body through food.
- Denaturation is the loss of a protein’s biological activity caused by changes in its secondary and tertiary structure caused by heat, pH changes, salts, and other factors.
- Enzymes are simple or conjugated proteins found in nature that catalyze biological reactions.
Vitamins
- Vitamins are nutrients that are required for normal health, growth, and nutrition.
- They are unable to be synthesized by organisms and must therefore be obtained through the diet.
- The vitamins A, D, E, and K are fat-soluble, whereas vitamins B and C are water-soluble.
Nucleic Acids
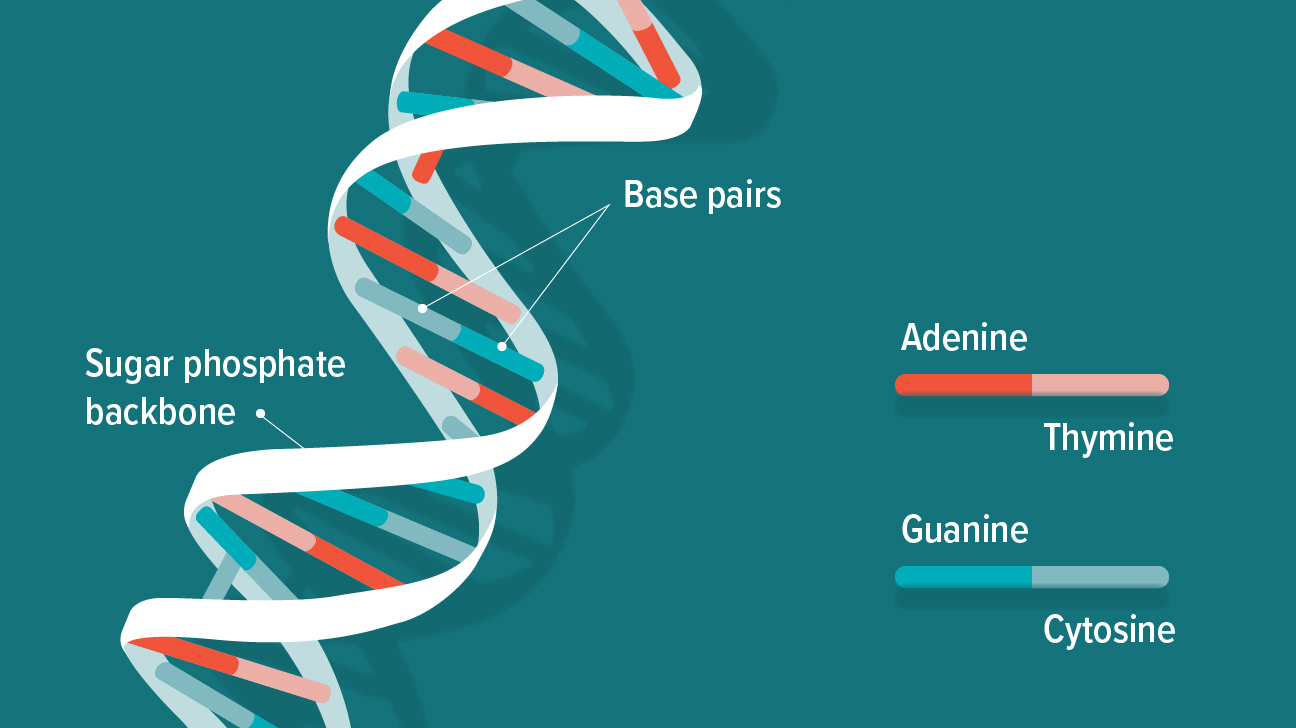
- Nucleic acids are polymers made up of nucleotides, which are complex repeating units. DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) are the two types of nucleic acids (ribonucleic acid).
- Replication and protein synthesis are two important functions of nucleic acids.
- A nucleotide is made up of three parts:
(a) A sugar unit with five carbon atoms, such as ribose or deoxyribose.
(b) A phosphate group
(c) a nitrogen-containing heterocyclic base.
- The nitrogen-containing bases in nucleotides are divided into two groups:
Purines: Adenine (A) and guanine (G)
Pyrimidines: The bases cytosine (C), thymine (T), and uracil (U)
- A mutation is a chemical change in the DNA molecule that can result in the production of proteins with different amino acid sequences.
- Changes in a DNA molecule can occur naturally or be triggered by radiation, chemicals, or viruses.