CBSE Class 12 Chemistry Chapter 8 Revision Notes
Chapter 8: The d and f Block Elements Revision Notes
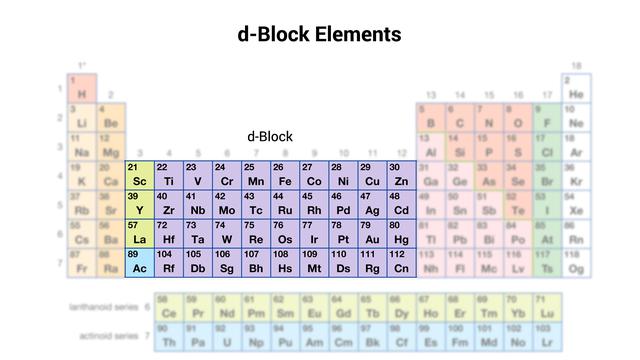
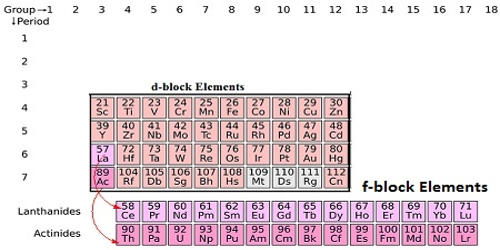
d-block or Transition Elements
- D-block or transition elements are elements that fall in the middle of the Periodic Table, between s-block and p-block elements (i.e., between groups 2 and 13).
- There are three series of transitions, each with ten elements.
- The first transition series entails the filling of three-dimensional orbitals. It progresses from scandium (Z = 21) to zinc (Z = 30).
- Filling of 4d-orbitals is involved in the second transition series. It begins with yttrium (Z=39) and ends with cadmium (Z=48).
- Filling of 5d-orbitals is the third transition series. Lanthanum (Z = 57) is the first element in this series. It is followed by the lanthanides, a group of 14 elements that involve the filling of 4f-orbitals. The third transition series includes the next nine elements, ranging from hafnium (Z = 72) to mercury (Z = 80).
- Inner-transition elements are the f-block elements.
- All transition elements are metallic in nature, good heat and electricity conductors, and show a gradual decrease in electropositive character as time passes.
- These metals are hard, have high densities, high enthalpies of atomisation, high melting and boiling points, and form alloys with other metals due to strong metallic bonds.
- As the series progresses, the melting point of the first increases to its maximum, then gradually decreases. The number of half-filled d-orbitals is roughly proportional to the strength of metallic bonds.
- The radii of ions in a given series with the same charge and magnitude decrease as the atomic number increases. This is due to the d-electrons’ poor shielding effect.
- Transition elements have higher ionization energies than s-block elements but lower ionization energies than p-block elements. In the series, it generally rises from left to right.
- Transition metals can be found in a wide range of oxidation states.
- The involvement of ns and (n – 1)d– electrons in bonding causes the variable oxidation states of transition metals.
- The majority of transition metals are electropositive enough. They produce H2 gas when they react with mineral acids.
- Transition elements are paramagnetic, as are many of their compounds.
- Another common property of transition metals is the formation of colored compounds (both in the solid state and in aqueous solution). This is due to the absorption of some visible light radiation, which causes d-d electron transitions in transition metal atoms.
- Transition elements, unlike s- and p-block elements, have the ability to form complexes. This is due to the fact that these elements (a) have small highly charged ions and (b) have d-orbitals that are vacant.
- In a variety of reactions, transition metals and their compounds act as catalysts.
- A large number of interstitial compounds are formed by transition metals.
- Transition metals are used to make a large number of alloys. It’s because their atoms in their metal crystal lattices can easily swap positions with each other.
- Transition metal oxides in lower oxidation states tend to be basic, while those in higher oxidation states tend to be amphoteric or acidic.
The f-block Elements
- The f-block elements are classified into two groups based on whether the last electron (differentiating electron) enters 4f- or 5f-orbitals, and are thus referred to as lanthanides or actinides.
- Actinides have a variety of oxidation states, with the + 3 oxidation state being the most common. Actinides have the highest oxidation state of + 7.19.
Properties of Lanthanides
- [Xe] 4f1-14 5d0-1 6s2 is the general electronic configuration.
- The metals have a silvery-white appearance.
- They are malleable, ductile, and have a low tensile strength, as well as being good heat and electricity conductors.
- They have a high melting point and have a relatively high density.
- The lanthanides have a + 3 oxidation state as their primary oxidation state. Some elements, however, have oxidation states of + 2 (Eu2+) and + 4 (Ce4+).
- The electronic transition between different 4 f-levels gives many lanthanide ions their color.
- The presence of unpaired electrons causes paramagnetism in the majority of lanthanide ions. The lanthanide ions with no 4f-electrons, such as La3+ and Ce4+, or with a completed 4f-level, such as Yb2+ and Lu3+, do not exhibit paramagnetism.
- Lanthanides tarnish easily in air and burn, forming trioxides (except cesium, which forms Ce02).
- Lanthanides’ oxides and hydroxides have a basic character.
- Lanthanide compounds are primarily ionic in nature.
- Lanthanoid contraction is the gradual decrease in atomic size across the first f- transition element series.
Properties of Actinides
- [Rn] 5f0-14 6ds0-1 7s2 is the general electronic configuration.
- All of the metals are silvery-white.
- The actinides have moderately high melting points.
- As the series progresses, the actinides’ ionic size decreases.
- The actinides can exist in a variety of oxidation states. In actinides, however, the +4 oxidation state is preferred.
- Some actinide elements, such as uranium, neptunium, and plutonium, can have a + 6 oxidation state.
- A significant number of actinide elements are radioactive. Beyond uranium, all elements are created by humans.
- Actinides are much more likely than lanthanides to form complexes.