Factors Affecting Ionization Energy Study Guide
Introduction
Taking care of children may be stressful at times! When you’re at the market with a little child, it could be a nightmare. The active kid must have a predisposition to stay still and keep consistency, but this is difficult to establish. A child’s attention may be drawn inadvertently to other powerful appealing forces such as a brilliant crayon, a fluffy toy, or something that has temporarily captured their interest.
In a similar way, the electrons in an atom are always in a state of battle. The ease with which an atom’s electrons may be removed has an impact on its reactivity. This property of electron removal is measured in terms of ionization energy.
WHAT IS IONIZATION ENERGY?
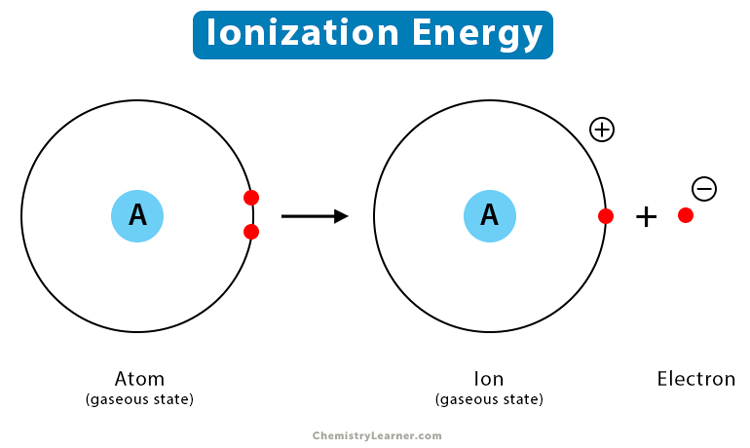
- Ionization enthalpy or ionization energy is the amount of energy necessary to remove the most loosely bonded electron from a solitary gaseous atom in order to generate a gaseous ion.
- It is expressed in kJ/mol, an energy unit similar to calories.
- The outermost valence electrons in any given atom will have lower ionization energies than the inner-shell electrons.
FACTORS AFFECTING IONIZATION ENERGY
1. SIZE OF THE ATOM:
- The distance between the nucleus and the electrons is inversely related to the attractive force connecting them.
- As a result, the further the electron is from the nucleus, the weaker the force of attraction on it is and the easier it is to remove it.
- As a corollary, as atomic size increases, the ionization energy drops.
- When we examine elements over time, we may discern a pattern in their ionization energy.
- The size of the atoms of the elements tends to decrease as we move along a period, resulting in a rise in ionization energy.
2. NUCLEAR CHARGE:
- The nucleus exerts a stronger pull on the valence shell electrons as the degree of nuclear charge increases.
- As a result, removing the valence shell electron becomes very difficult.
- Therefore, as the nuclear charge grows, so does the ionization energy.
3. ELECTRONIC CONFIGURATION:
- The value of an atom’s ionization energy may be affected by its electronic configuration.
- Shells that are half-full and totally filled are proven to be more stable.
- Because it is difficult to remove an electron from atoms with entirely filled shells, they are considered to have a stable electronic state, resulting in high ionization energy.
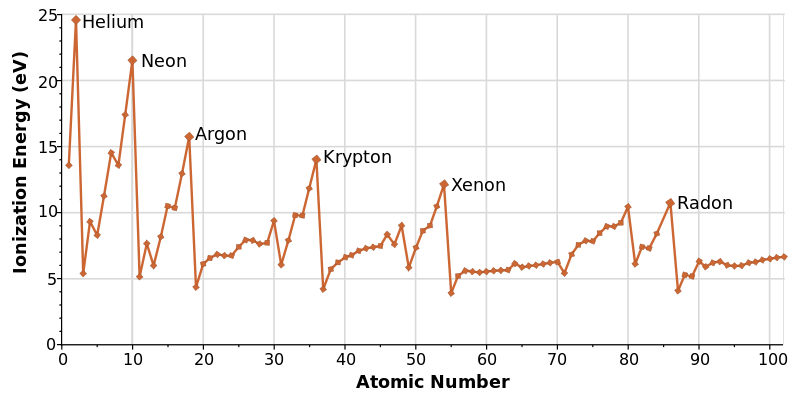
IONIZATION ENERGY TREND IN THE PERIODIC TABLE:
GENERAL PERIODIC TRENDS
- When descending from top to bottom in a group, ionization energy decreases.
- Ionization energy increases from left to right across a period.
Conclusion
- Ionization enthalpy or ionization energy is the amount of energy necessary to remove the most loosely bonded electron.
- When descending from top to bottom in a group, ionization energy decreases.
- Ionization energy increases from left to right across a period.
FAQs
1. What is the trend for ionization energy going down?
As you proceed down the periodic table, the first ionization energy often drops. Because the outermost electron is further away from the nucleus on average, it is boundless securely and needs less energy to remove.
2. Why does ionization energy change across a period?
The initial ionization energy increases as we travel left to right across a period on the periodic table. This is because when the nuclear charge rises, the outermost electron becomes more tightly connected to the nucleus.
3. What does a periodic trend look like on the periodic table?
The periodic table is grouped and organized with a certain pattern or regular modification of an element’s characteristics as the atomic number increases, which is known as the periodic trend. As a result, periodic trends resemble a pattern in the periodic table.
We hope you enjoyed studying this lesson and learned something cool about Factors Affecting Ionization Energy! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise, it makes studying much more fun!😎
SOURCES
- Periodic Trends: https://www.ck12.org/c/chemistry/periodic-trends%3A-ionization-energy/lesson/Periodic-Trends-Ionization-Energy-CHEM/ accessed 16 march 2022
- Ionization Energy: https://byjus.com/chemistry/periodic-trends-in-ionisation-enthalpy-of-elements accessed 16 march 2022