Hybrid Orbitals – sp³ Study Guide
INTRODUCTION
When we were little kids 🧒, we all stared up at the night sky 🌃 with amazement 😮. The bright spots somehow gave a soothing relief, a calm. We fantasized about the stars and space, and we often slept 😴 counting stars. We had so many questions! We often need to view the world around us in new ways to comprehend things better. Hybridization is one such example where we need to expand our normal horizon of thinking.
HYBRIDIZATION
When two atomic orbitals unite to produce a hybrid orbital in a structure, the energy of individual atoms’ orbitals is redistributed to give orbitals of comparable energy. Hybridization is the term for this process.
The atomic orbitals with equivalent energies are blended together during the hybridization process. It usually includes the merging of two ‘s’ orbitals or 2 ‘p’ orbitals or combining an ‘s’ orbital with a ‘p’ orbital, as well as a’s’ orbital along with a ‘d’ orbital. Hybrid orbitals are the novel orbitals that result from this process. Hybrid orbitals are particularly valuable in describing atomic bonding characteristics and molecule geometry.
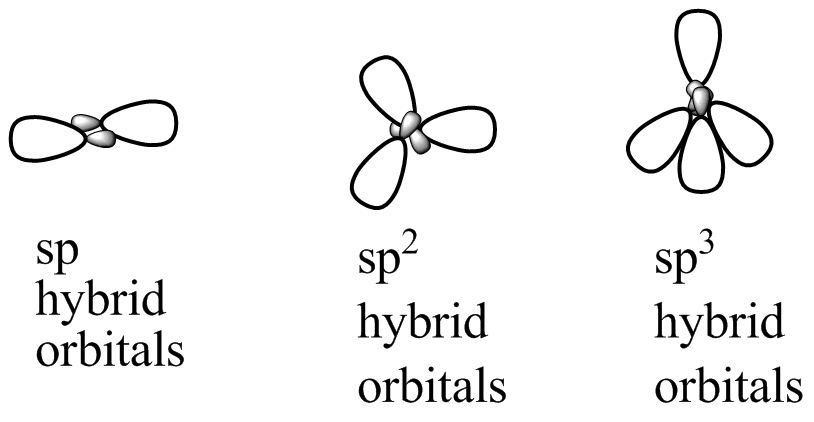
sp³ HYBRIDIZATION
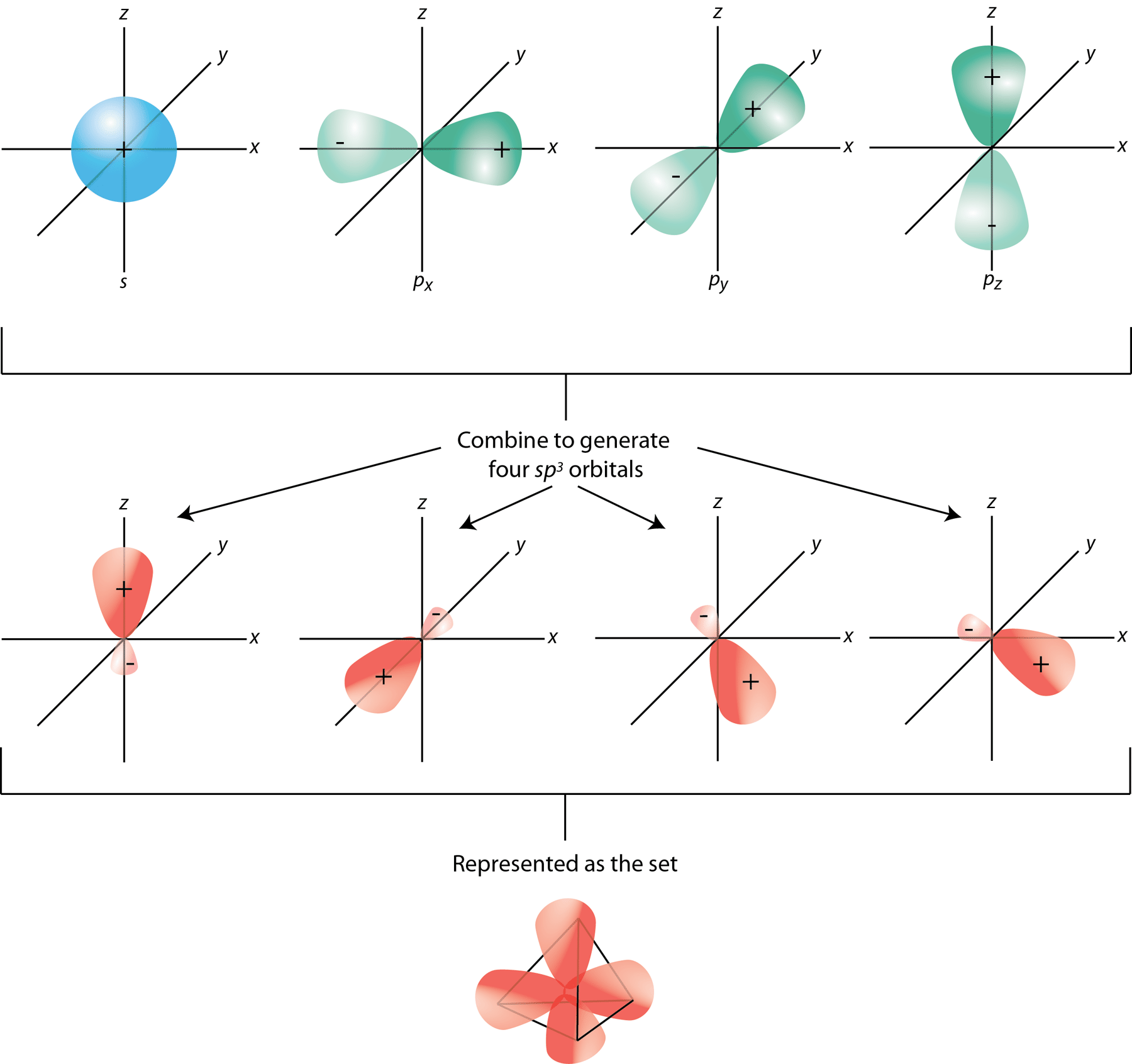
A tetrahedral hybridization, or sp³, occurs whenever 1’s’ orbital and three ‘p’ orbitals corresponding to much the same shell of an atom combine to generate four new equivalent orbitals. sp³ hybrid orbitals are the new orbitals that have been generated.
- The 3 p atomic orbitals are perpendicular at a 90-degree angle. The apparent HCH bond angle in the tetrahedral CH₄ compound is 109.5, as shown by the valence shell electron pair repulsion (VSEPR) theory. As a result, the methane molecules cannot be accurately described by a simple overlapping of carbon’s 2s and 2p orbitals with every hydrogen atom’s 1s orbitals.
- These are aimed at the four corners of a conventional tetrahedron and form a 109° angle with each other.
- The sp³ hybridization orbitals have a 109.280-degree angle between them.
- Each sp³ hybridized orbital has a 25% s component and 75% p character.
CONCLUSION
- The mingling of atomic orbitals in an atom to form a hybrid orbital is known as hybridization.
- A tetrahedral hybridization, or sp³, occurs whenever 1 ‘s’ orbital and three ‘p’ orbitals corresponding to much the same shell of an atom combine to generate four new equivalent orbitals.
FAQs:
1. What are sp² and sp³ in chemistry?
One s and 2 p atomic orbitals are mixed in sp² hybridization, and one s and 3 p are mixed in sp³ hybridization.
2. What type of bond is sp³?
Four equal σ bonds make up the sp³ molecule. Two s and three 2p orbitals unite to form four identical orbitals, usually known as sp³ hybrids, during hybridization.
3. What is hybridization in chemistry?
In valence bond theory, orbital hybridization combines atomic orbitals to generate new hybrid orbitals. They have different energies, shapes, and other properties than the constituent atomic orbitals, and they are suited for electron pairing to form chemical bonds.
We hope you enjoyed studying this lesson and learned something cool about Hybrid Orbitals – sp³! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our app to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES:
- Hybrid Orbitals – sp3. https://www.ck12.org/c/chemistry/hybrid-orbitals-sp3/lesson/Hybrid-Orbitals-sp3-CHEM/. Accessed 18 Feb 2022.
- Sp3 Hybridization. https://courses.lumenlearning.com/introchem/chapter/sp3-hybridization/. Accessed 18 Feb 2022.
- Hybridization. https://byjus.com/jee/hybridization/. Accessed 18 Feb 2022.