Polyatomic Ions – Naming and Formulas Study Guide
INTRODUCTION
Did you know that it takes about three and a half hours to read the human protein’s chemical name Titin, which has 1,89,819 letters? So, it is difficult to write the chemical names, but with some rules, we can make it easy. Also, let’s study what ‘ite’ and ‘ate’ mean in chemistry. So, let’s make complicated things easy!
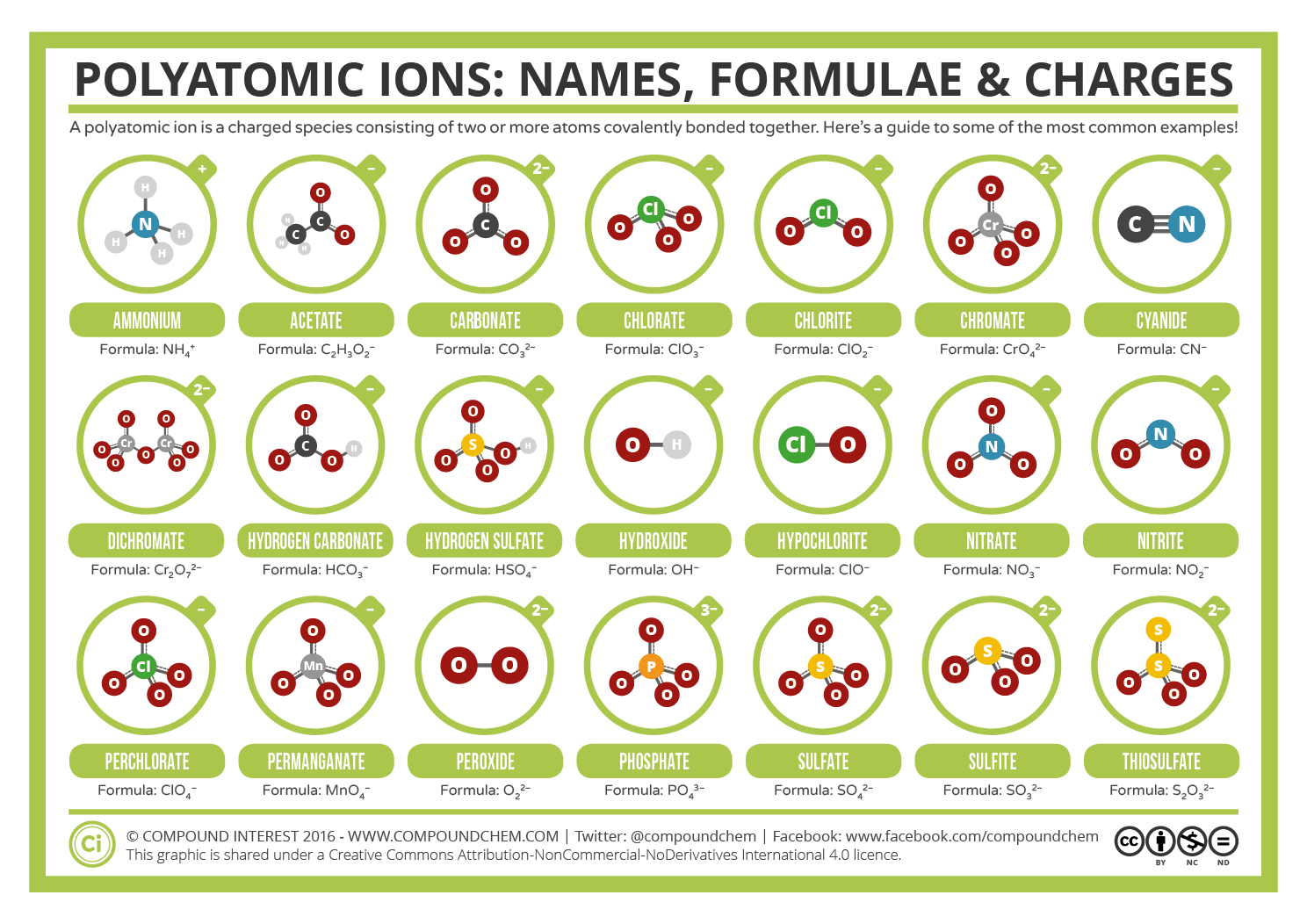
POLYATOMIC IONS
Poly means many. So, polyatomic ions are ones that have more than one atom. Na (sodium) is a mono-atomic ion, while NH4 (ammonium) is a polyatomic ion as it has 5 atoms in total. Some more examples of polyatomic ions are carbonate(CO₃²⁻), hydroxide(OH-), phosphate (PO₃⁴⁻), hypochlorite (OCl-), etc. The 1 in NH₄ ion is the total charge on the ammonium ion, while -2 in CO₃²⁻ is the total charge present on all the atoms collectively.
Polyatomic ions are present everywhere:
- NaHCO₃, sodium bicarbonate, is used in cooking as well as an antacid.
- NaHCO₃, sodium bicarbonate, is used in cooking as well as an antacid.
Polyatomic ions are formed by different atoms joined together by covalent bonds. Generally, there are more common polyatomic anions than cations. Some common polyatomic ions are:
NAMING
For polyatomic ions such as OH-, the word hydroxide is used. Generally, the ‘ide’ suffix is used for mono-atomic ions, for example, chloride for Cl-.
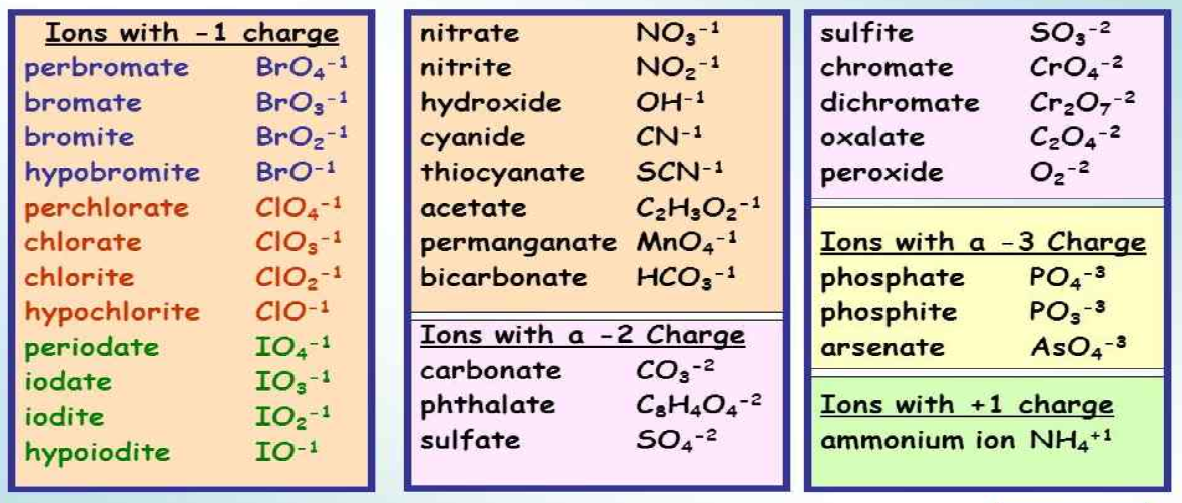
For polyatomic ions, ‘ate’ or ‘ite’ suffixes are commonly used. For example, NO3- and NO₂- are named as nitrite and nitrate, respectively. Also, ClO₂- is called chlorite and ClO₃- as chlorate. These are examples of oxoanions. The oxoanion with fewer O atoms is given the suffix ‘ite’ while the one with more O atoms is given ‘ate.’ ClO- hypochlorite, ClO₂- chlorite, ClO₃- chlorate, ClO₄- perchlorate
In these cases, the ion with more O atoms is given the prefix ‘per’ as ClO4- perchlorate, and the one with fewer O atoms than its ‘ite’ counterpart is given the prefix ‘hypo’ as ClO- hypochlorite.
CONCLUSION
- Polyatomic ions are the ones that have more than one atom. For example, NH₄ (ammonium).
- The oxoanion with fewer O atoms is given the suffix ‘ite’ while the one with more O atoms is given ‘ate.’
- The ion with more O atoms is given the prefix ‘per’ as ClO₄- perchlorate, and the one with fewer O atoms than its ‘ite’ counterpart is given the prefix ‘hypo’ as ClO- hypochlorite.
FAQs:
1. What is a polyatomic ion?
Polyatomic ions are the ones that have more than one atom.
2. Out of NO₃- and NO₂- which one is nitrite?
NO₂- is nitrite as it has fewer O atoms.
3. What is the total charge on NH₄ ions?
The total charge on NH₄ ions is 1.
We hope you enjoyed studying this lesson and learned something cool about Polyatomic Ions and Naming! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise it makes studying much more fun! 😎
SOURCES
- Polyatomic Ions: https://www.ck12.org/c/chemistry/polyatomic-ions-naming-and-formulas/lesson/Polyatomic-Ions-CHEM/.Accessed 7th March 2022.
- Polyatomic Ions: https://www.khanacademy.org/science/chemistry/atomic-structure-and-properties/names-and-formulas-of-ionic-compounds/a/polyatomic-ions. Accessed 7th March 2022.
- Ionic Compounds: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_A_Molecular_Approach_(Tro)/03%3A_Molecules_Compounds_and_Chemical_Equations/3.05%3A_Ionic_Compounds-_Formulas_and_Names Accessed 7th march 2022.