Valence Electrons In Beryllium Study Guide
Introduction
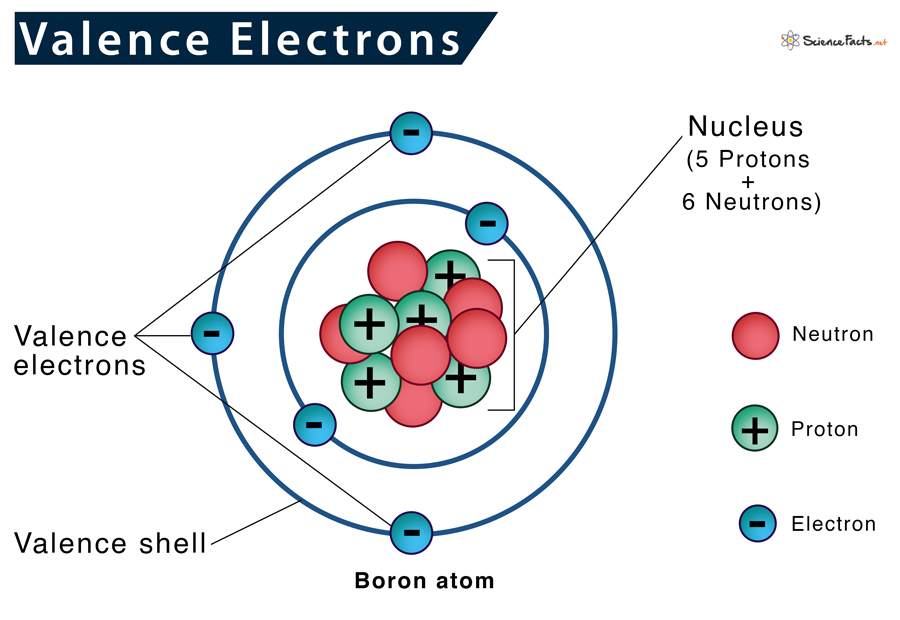
What is a valence electron? Valence electrons are the electrons in the outermost shell of an atom, while the electrons which are mostly present within the inner circle are known as core electrons. Lewis structure helps us determine valence electrons and how it helps any chemical reaction and predict the type of bond.
What Exactly Are Valence Electrons?
You’d find valence electrons at the outermost shell of an atom. These electrons are relatively loosely placed in the atom as they are furthest from the nucleus; therefore, they participate in various chemical reactions and form bonds. The number of valence electrons varies for different elements, which determines what the electronegativity reactivity would be, along with the number of bonds it could form.
The term valence stands for the ability of any element to form bonds with other elements and their atoms. At first, the valence of an atom was determined by checking how many hydrogen atoms it could bond to. An example would be NH3 which has a valence of 3; therefore, the number of valence electrons would be 3.
What Are The Notable Characteristics Of Valence Electrons?
Electrons, as we know by now, are involved in various reactions as well as chemical bonding. A quick hack to find the number of valence electrons present in an atom, is through the periodic table! The number of valence electrons = the atomic group number. Atoms are much more stable when they have a filled valence shell of electrons.
What are some of the key characteristics of valence electrons?
- For main group elements, the valence electrons are only located at the outermost electron shell.
- For a transition metal, valence electrons could exist in the inner shell.
- An atom would be chemically inert if it consists of a closed shell of valence electrons.
- A valence electron either releases or absorbs energy in the form of a photon.
Example: The Valence Electrons Of Beryllium
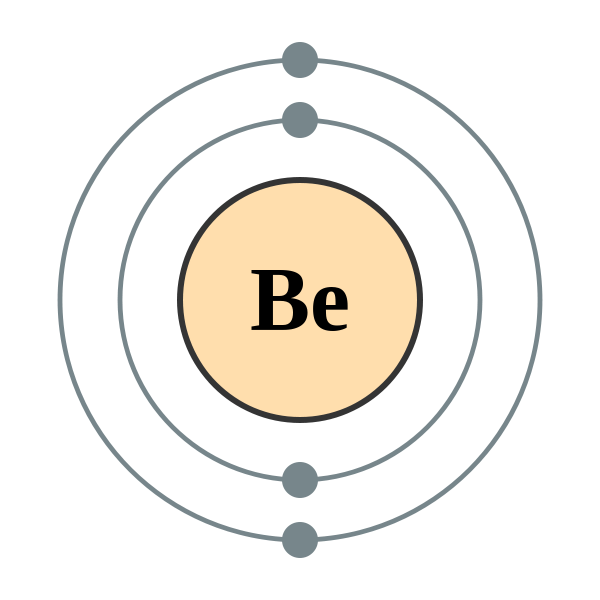
The total number of electrons in the last shell after doing the electron configuration of Beryllium is its valence electron number. How can we calculate the valence electron Of Beryllium?
- First figure out the total electron number in Beryllium. For this, you have to know the total proton number of Beryllium as well. As we all know by now, electron number = proton number; therefore, check the periodic table, and you would find that the atomic number of Beryllium is 4. Hence, Beryllium has a total of four electrons.
- You have to do the electron configuration then where Beryllium would come down to 1s2 2s2.
- Next, identify the valence shell. According to electron configuration, it shows that Beryllium has two electrons in the last orbit; therefore, the valence electron would be 2.
Conclusion
- The total number of protons equals the total number of electrons.
- The last shell of the atomic orbital is known as the valence shell.
- The electronic configuration of Beryllium comes down to 1s2 2s2; hence, the Beryllium valence electrons would be 2.
FAQs
-
How Many Valence Electrons Does Beryllium Lose?
Beryllium could lose 2 valence electrons.
-
Does Beryllium Have 6 Valence Electrons?
No, Beryllium does not contain 6 valence electrons. Beryllium has 2 valence electrons.
We hope you enjoyed studying this lesson and learned something cool about Valence Electrons in Beryllium! Join our Discord community to get any questions you may have answered and to engage with other students just like you! Don’t forget to download our App to experience our fun VR classrooms – we promise it makes studying much more fun😎
REFERENCE
- What Are Valence Electrons? https://chemistrytalk.org/what-are-valence-electrons/. Accessed 7th March 2022.
- Valence Electrons: https://byjus.com/chemistry/valence-electrons/. Accessed 7th March 2022.
- Valence Electrons of Beryllium: https://valenceelectrons.com/valence-electrons-of-beryllium/. Accessed 7th March 2022.